An alkane (molecular weight = 86) on monochlorination gives only two monochloro derivatives. One isomer (A) of this alkane is obtained on catalytic reduction of alkene (B) which gives four monochloro derivatives. Structure of (A) is
A
CH3−CH2−CH−CH3|CH2−CH3
B
CH3− CH∣ CH3−CH2−CH2−CH3
C
CH3−CH2−CH3∣C∣CH3−CH3
D
CH3−C∣CH3H−C∣CH3H−CH3
Text Solution
The correct Answer is:A
Molecular weight 86 overset"Monochlorination"to ubrace(I+II)_("isomers")
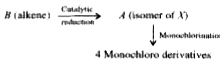
1↓CH3−2↓CH2−3↓C∣CH3↑4H−CH2CH3
As A is isomer of X thus it should have molecular formula C6H14. This isomer of hexane gives four monochloro products on chlorination
|
Similar Questions
Knowledge Check
An alkane of molecular weight 86 g/mol on monochlorination gives two products. The alkane is
A2 - MethylbutaneBn - butaneC2, 2- Dimethyl propaneD2, 3- Dimethyl butaneAn alkane by molecular weight 72 upon chlorination gives one monochlorination product. The alkane is
A2-MethylbutaneBn-PentaneC2,2-DimethylpropaneDAll of theseAn alkane with molecular weight 72 upon chlorination gives only one monochlorination product. The alkane is
A2-methylbutaneB11-pentaneC2,2-dimethylpropaneDall of these.
An alkane (mol. Wt. = 86) on bromination gives only two monobromo derivatives (excluding stereoisomers). The alkane is :
View SolutionAn alkane (mol. Wt. = 86) on bromination gives only two monobromo derivatives (excluding stereoisomers). The alkane is:
View SolutionWhich one of the following gives only one monochloro derivative?
View SolutionWhich one of the following gives only one monochloro derivative?
View SolutionAn alkane (mol. Wt.=86) on bromination gives only two monobromo derivatives (exluding stereoisomers) the alkane is:
View Solution

