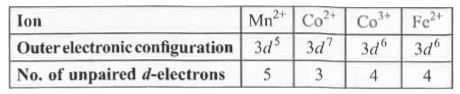
Which of the following contains maximum number of unpaired electrons?
A
Mn2+
B
Co3+
C
Co2+
D
Fe2+
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
- THE d-AND f- BLOCK ELEMENTSBOOK - MTG GUIDECHAPTER - THE d-AND f- BLOCK ELEMENTSEXERCISE - CHECK YOUR NEET VITALS
10 Videos
- THE d-AND f- BLOCK ELEMENTSBOOK - MTG GUIDECHAPTER - THE d-AND f- BLOCK ELEMENTSEXERCISE - CHECK YOUR NEET VITALS
10 Videos
- SURFACE CHEMISTRYBOOK - MTG GUIDECHAPTER - SURFACE CHEMISTRYEXERCISE - AIMPT/NEET MCQS
11 Videos
- THE p-BLOCK ELEMENTS (GROUP 15 TO 18)BOOK - MTG GUIDECHAPTER - THE p-BLOCK ELEMENTS (GROUP 15 TO 18)EXERCISE - CHECK YOUR NEET VITALS
14 Videos
Similar Questions
Knowledge Check
Which of the following contains the maximum number of unpaired electrons?
AMn2+BCo3+CCo2+DFe2+Which of the following contains the maximum number of unpaired electrons ?
AMn2+BCo3+CCo2+DFe2+Which of the following contains the maximum number of unpaired electrons?
AMn2+BCo3+CCo2+DFe2+
Which of the following contains the maximum number of unpaired electrons?
View SolutionWhich one of the following contains maximum
number of unpaired electrons in antibonding molecular orbitals ?
View SolutionWhich of the following has maximum number of unpaired electrons?
View SolutionWhich of the following has maximum number of unpaired electrons ?
View SolutionWhich of the following has the maximum number of unpaired electrons?
View Solution