Which of the following is arranged in order of decreasing thermal stability?
A
Zn>Hg>Cd
B
Cd>Hg>Zn
C
Zn>Cd>Hg
D
Hg>Cd>Zn
লিখিত জবাব
The correct Answer is:C
Melting point of Zn,Cd, and Hg follows the order Zn>Cd>Hg.
Higher the m.pt . Higher will be the thermal stability. Hence order of thermal stability is Zn>Cd>Hg.
|
টপার এই প্রশ্নগুলো সমাধান করেছে
- THE d-AND f- BLOCK ELEMENTSBOOK - MTG GUIDECHAPTER - THE d-AND f- BLOCK ELEMENTSEXERCISE - CHECK YOUR NEET VITALS
10 Videos
- THE d-AND f- BLOCK ELEMENTSBOOK - MTG GUIDECHAPTER - THE d-AND f- BLOCK ELEMENTSEXERCISE - CHECK YOUR NEET VITALS
10 Videos
- SURFACE CHEMISTRYBOOK - MTG GUIDECHAPTER - SURFACE CHEMISTRYEXERCISE - AIMPT/NEET MCQS
11 Videos
- THE p-BLOCK ELEMENTS (GROUP 15 TO 18)BOOK - MTG GUIDECHAPTER - THE p-BLOCK ELEMENTS (GROUP 15 TO 18)EXERCISE - CHECK YOUR NEET VITALS
14 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Correct order of decreasing thermal stability is
ANH3>PH3>AsH3>SbH3BPH3>NH3>AsH3>SbH3CAsH3>PH3>NH3>SbH3DSbH3>AsH3>PH3>NH3Which of the following shows the correct order of decreasing stability?
A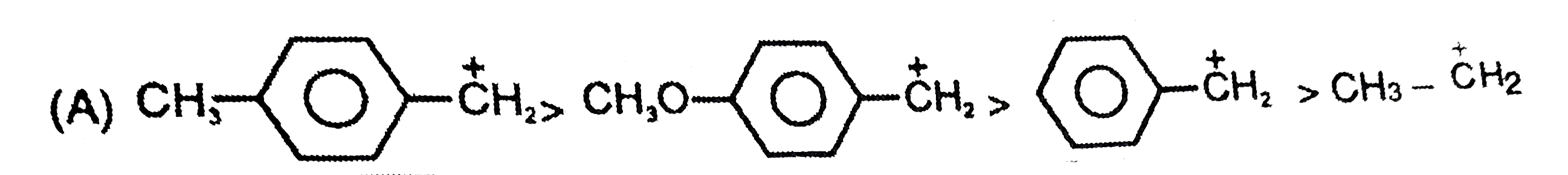 B
B C
C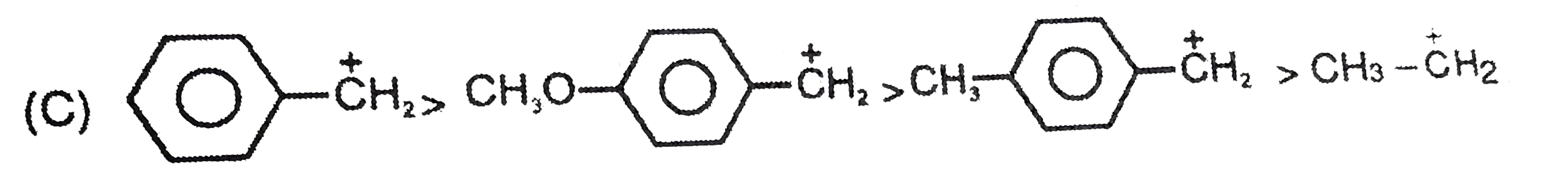 D
D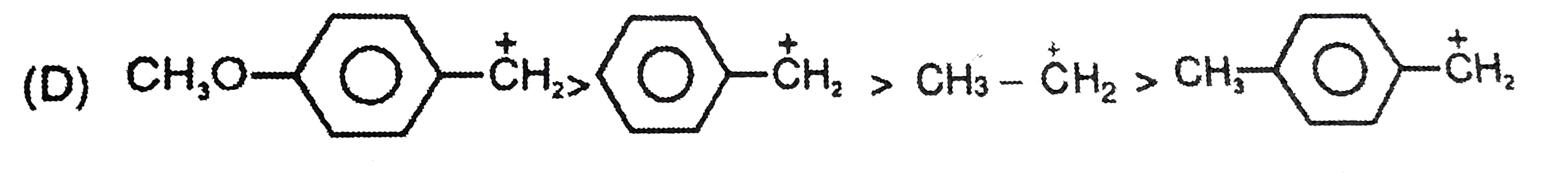
Arrange the following conformations of ethane in the order of decreasing stability
AEclipsed > Staggered > SkewedBEclipsed > Skewed > StaggeredCStaggered > Eclipsed > SkewedDStaggered > Skewed > Eclipsed
Arrange the following conformations of ethane in the order of decreasing stability
View SolutionArrange the following sulphates of alkaline earth metals in order of decreasing thermal stability :
BaSO4,MgSO4,CaSO4,SrSO4.
View SolutionArrange the following sulphates of alkaline earth metals in order of decreasing thermal stability: BeSO4,MgSO4,CaSO4,SrSO4.
View SolutionArrange the following sulphates of alkaline earth metals in order of decreasing thermal stability: BeSO4,MgSO4,CaSO4,SrSO4.
View SolutionWhich of the following is not the correct order of thermal stability?
View Solution

