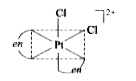
Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
cis-isomer of the complex [Pt(en)2Cl2]2+ is optically active.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - MTG GUIDECHAPTER - COORDINATION COMPOUNDSEXERCISE - NEET CAFÉ (WERNER.S THEORY, SOME IMPORTANT TERMS AND NOMENCLATURE OF COORDINATION COMPOUNDS)
18 Videos
- COORDINATION COMPOUNDSBOOK - MTG GUIDECHAPTER - COORDINATION COMPOUNDSEXERCISE - NEET CAFÉ IMPORTANCE AND APPLICATIONS OF COORDINATION COMPOUNDS)
7 Videos
- CHEMISTRY IN EVERYDAY LIFE BOOK - MTG GUIDECHAPTER - CHEMISTRY IN EVERYDAY LIFE EXERCISE - AIPMT/NEET
9 Videos
- ELECTROCHEMISTRYBOOK - MTG GUIDECHAPTER - ELECTROCHEMISTRYEXERCISE - AIPMT/NEET(MCQs)
5 Videos
Similar Questions
(a) For the complex [Fe(CN)6]3− , write the hybridisation type, magnetic character and spin nature of the complex .(At. Number : Fe=26)
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active .
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
View Solution(a) For the complex [Fe(CN)6]3− , write the hybridisation type, magnetic character and spin nature of the complex .(At. Number : Fe = 26)
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active .
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive.
View Solution(a) For the complex [Fe(H2O)6]3+ , write the hybridization, magnetic character and spin of the complex. (At. Number : Fe =26)
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive.
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive . Also write the name of this entity according to the IUPAC nomenclature .
View Solution(a) For the complex [Fe(H2O)6]3+ , write the hybridization, magnetic character and spin of the complex. (At. Number : Fe=26)
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive.
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl]2+ which is optically inactive. Also write the name of this entity according to the IUPAC nomenclature.
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl]2+ which is optically inactive. Also write the name of this entity according to the IUPAC nomenclature.
View SolutionDraw the geometrical isomers of complex [Pt(en)2Cl2]2+ .
View Solution

