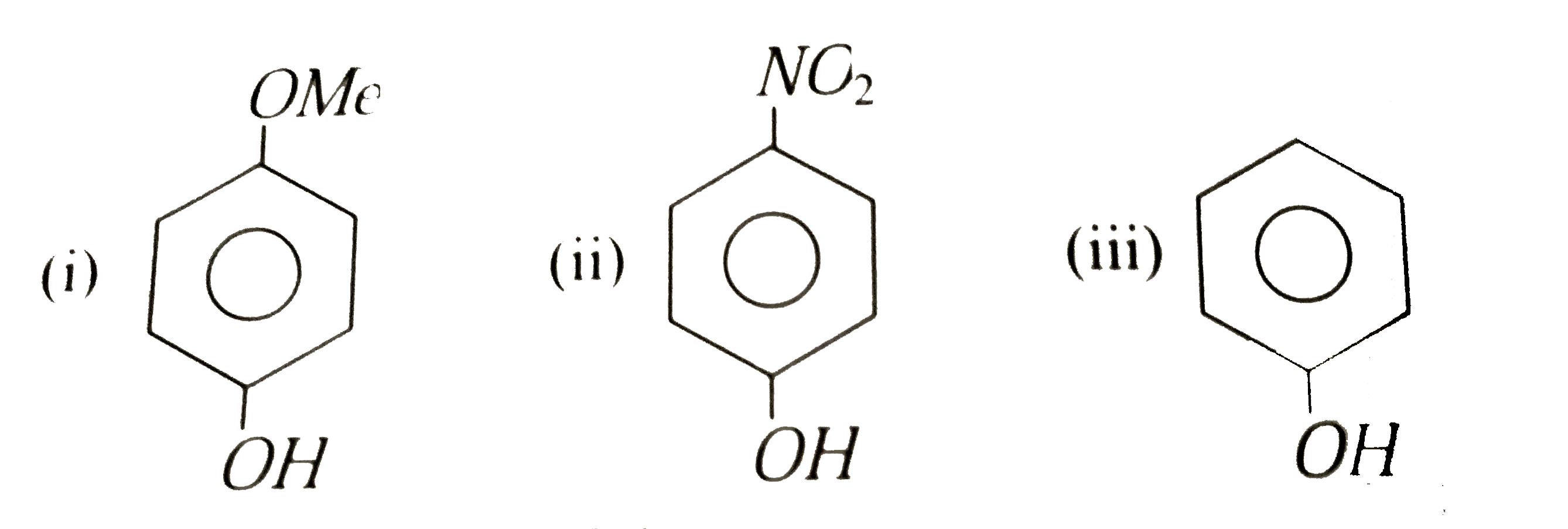
Given are cyclohexanol (I), acetic acid (II), 2,4,6-trinitrophenol (III) and phenol (IV). In these the order of decreasing acidic character will be
A
III>II>IV>I
B
II>III>I>IV
C
II>III>IV>I
D
III>IV>II>I
Text Solution
The correct Answer is:A
Since phenols and carboxylic acids are more acidic than aliphatic alcohols, we find that cyclohexanol (I) is least acidic.
Out of the two given phenols, III is more acidic than IV. This is because of the presence of three highly electron withdrawing −NO2 groups on the benzene ring which makes the O−H bond extremely polarized. This facilitates the release of H as H+. Thus, III>IV.
In acetic acid the electron withdrawing −O∣∣C− in the −COOH group polaries the O−H bond and increases the acidic strength. Acetic acid is therefore more acidic than phenol or cyclohexanol.
∴ The order is III>II>IV>I.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Given are cyclohexanol (I), acetic acid (II),2,4,6−trinitrophenol (III) and phenol (IV). In these the order of decreasing acidic character will be:
AIIIgtIVgtIIgtIBIIIgtIIgtIVgtICIIgtIIIgtIgtIVDIIgtIIIgtIVgtICyclohexanol (I), acetic acid (II), 2,4,6-trinitrophenola(III) and phenol(IV) are given. In these the order of decreasing acidic character will be.
AIII>II>IV>IBII>III>I>IVCII>III>IV>IDIII>IV>II>IThe decreasing order of acidic character is :
A(I)>(II)>(III)>(IV)B(I)>(II)>(IV)>(III)C(IV)>(III)>(II)>(I)D(III)>(IV)>(II)>(I)
The decreasing order of the acidic character is :
 .
.
View SolutionAmong ethanol (I), acetic acid (II), phenol (III) and benzoic acid (IV), the correct order of increasing acid strength is
View SolutionAmong ethabol (I) Acetic acid (II) phenol (III) and Benzoic acid (IV) the correct order of increasing acid strength is
View SolutionGiven
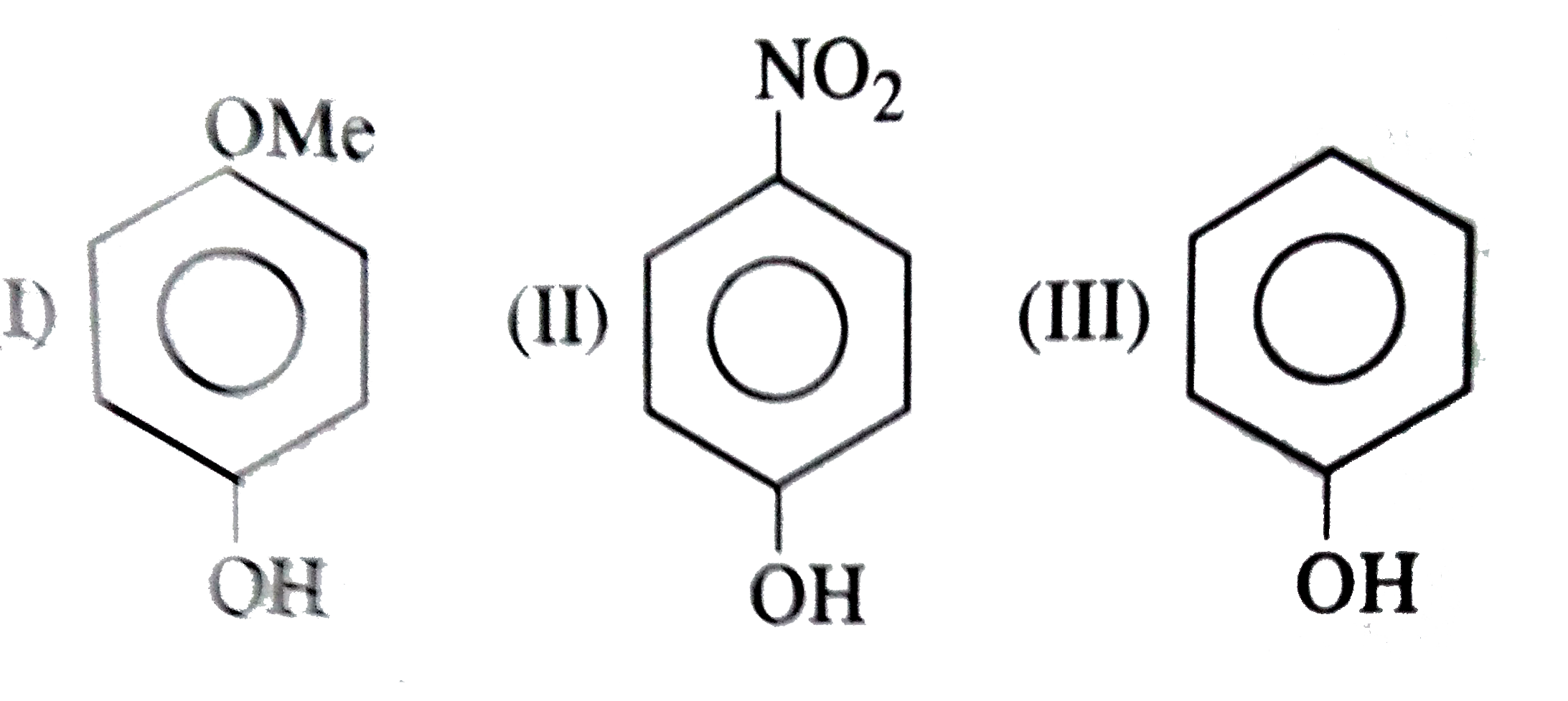
The decreasing order of their acidic character is
View SolutionAmong phenol (I), o-nitrophenol (II), p-nitrophenol (III),m-nitrophenol (IV), the correct order of decreasing acid strength is
View Solution