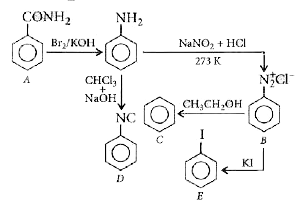
An aromatic compound 'A' of molecular formula C7H7ON undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions :
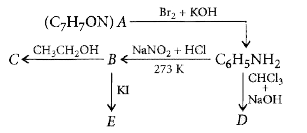
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING NITROGENBOOK - MTG GUIDECHAPTER - ORGANIC COMPOUNDS CONTAINING NITROGENEXERCISE - NEET CAFE (TOPICWISE PRACTICE QUESTIONS)
140 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBOOK - MTG GUIDECHAPTER - ORGANIC COMPOUNDS CONTAINING NITROGENEXERCISE - CHECK YOUR NEET
25 Videos
- HALOALKANES AND HALOARENES BOOK - MTG GUIDECHAPTER - HALOALKANES AND HALOARENES EXERCISE - AIMPT/NEET
10 Videos
- POLYMERSBOOK - MTG GUIDECHAPTER - POLYMERSEXERCISE - AIPMT/NEET
15 Videos
Similar Questions
Knowledge Check
An aromatic compound has molecular formula C7H8O. The number of phenolic isomers for this compound is
A4B3C5D6An optically active compound A with molecular formula C8H14 undergoes catalytic hydrogenation togive meso compound, the structure of (A) is:
A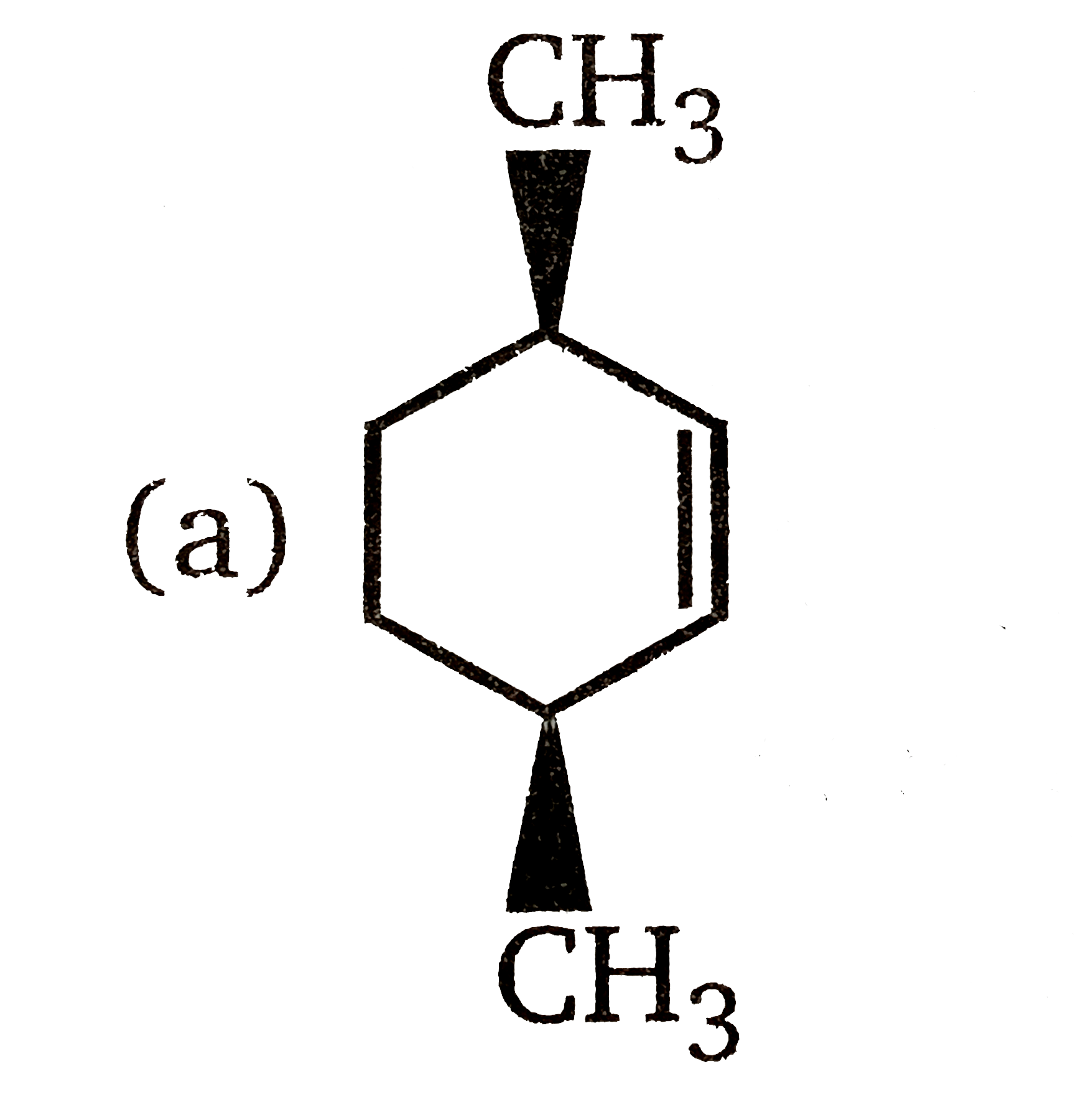 B
B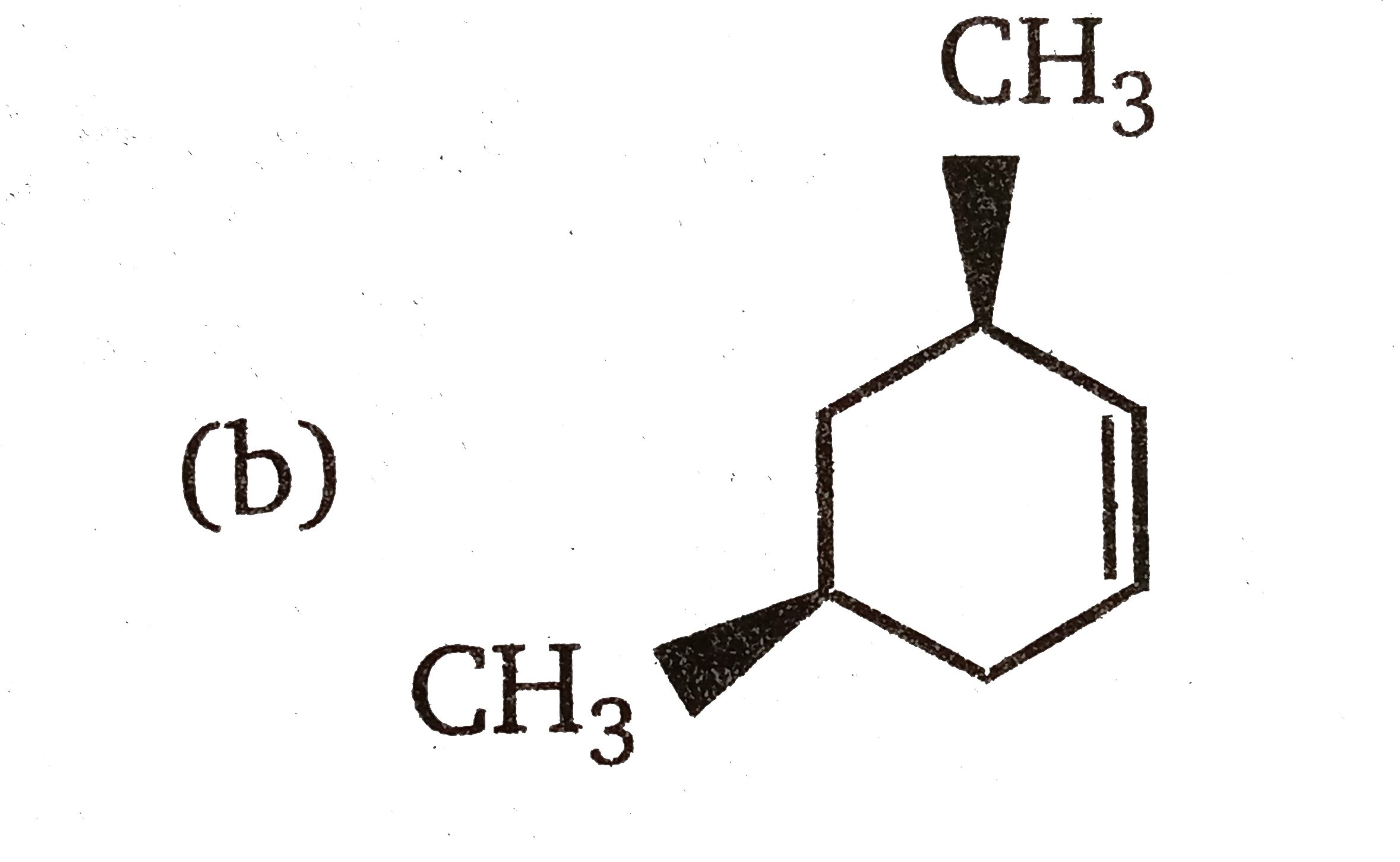 C
C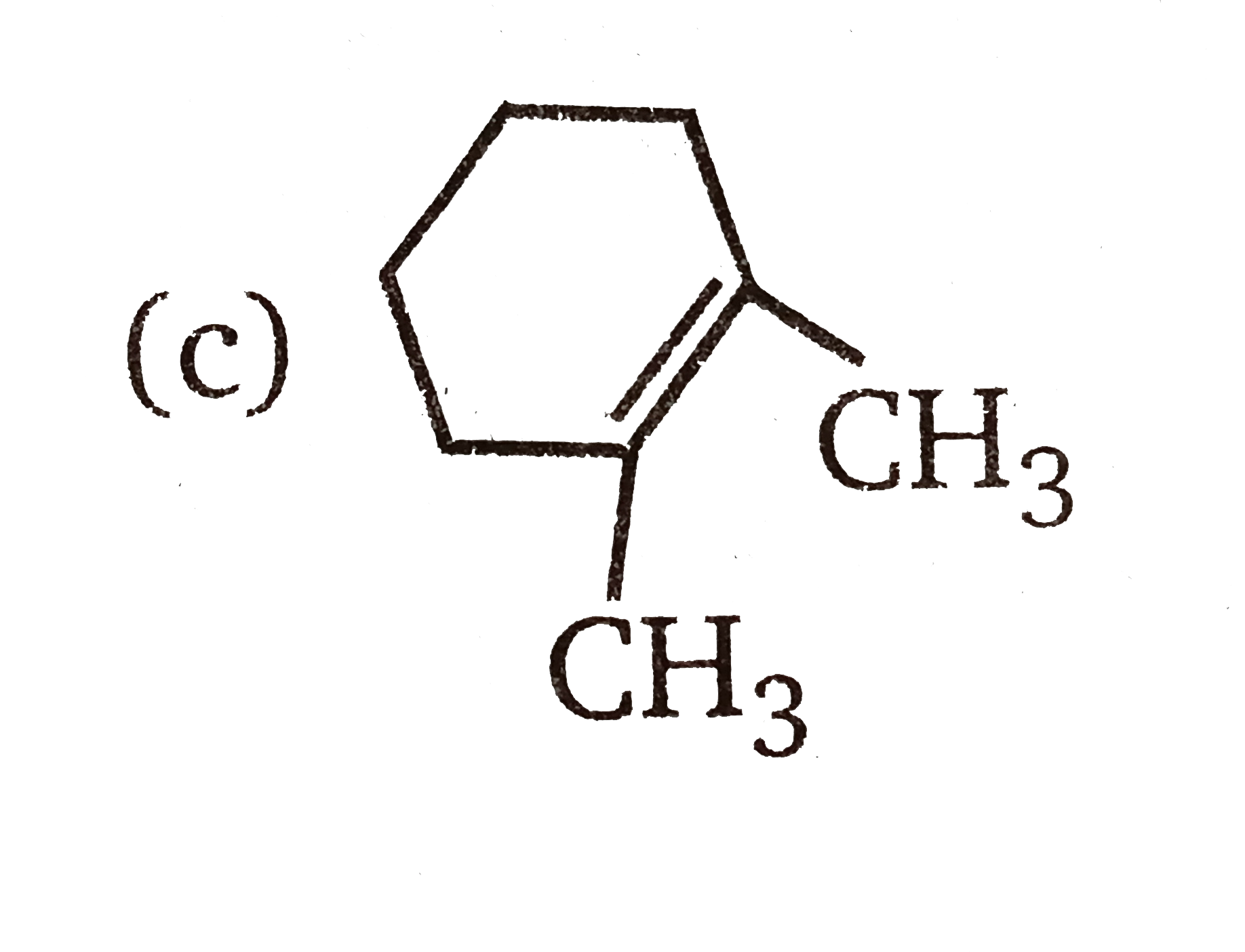 D
D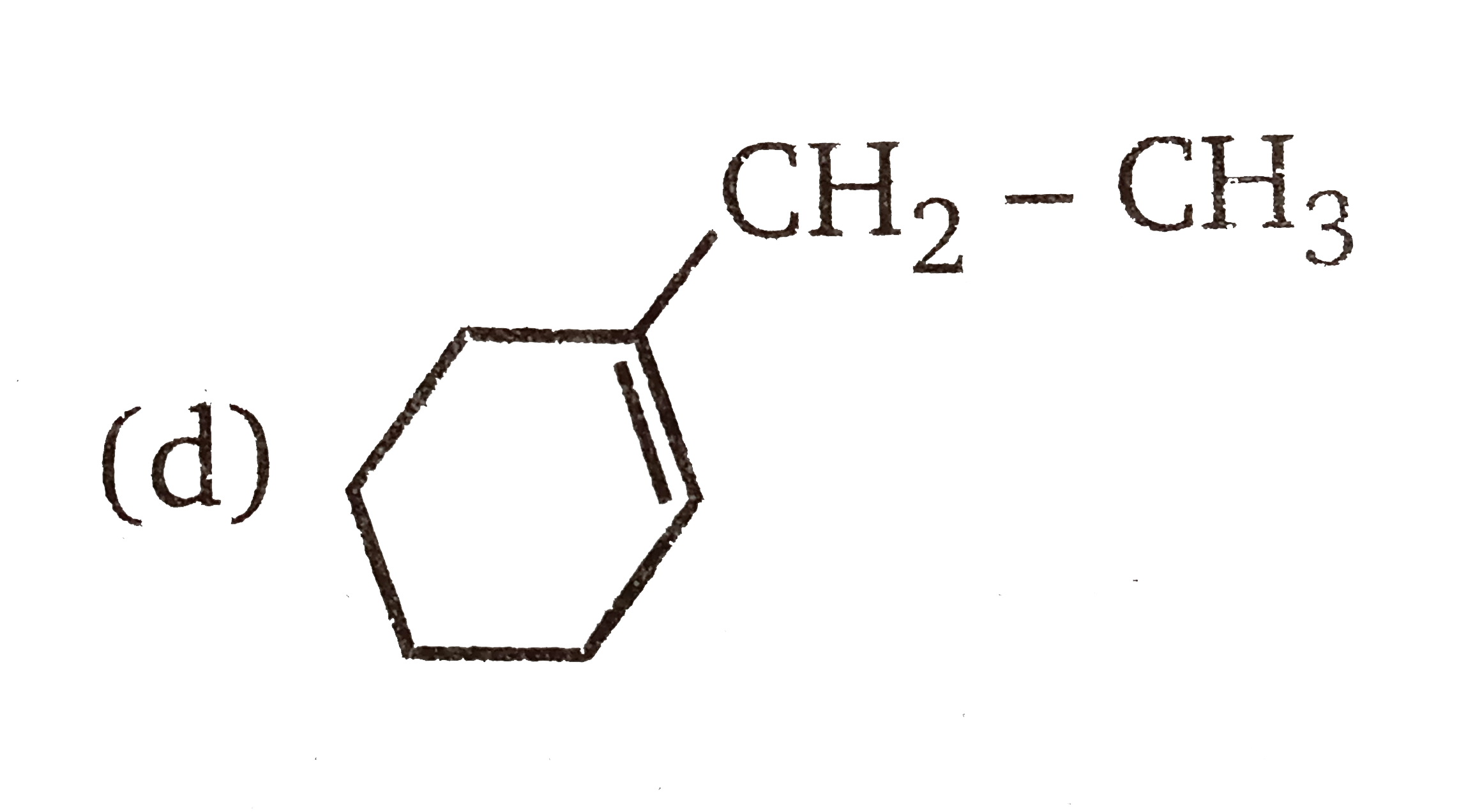
An aromatic compound A of the molecular formula C8H10O on reaction with iodine and dilute NaOH gives a yellow precipitate . The structure of the compound is expected to be:
A BC6H5CHOHCH3C
BC6H5CHOHCH3C D
D
An aromatic compound 'A' having molecular formula C7H6O2 on treating with aqueous ammonia and heating forms compound 'B'. The compound 'B' on reaction with molecular bromine and potassium hydroxide provides compound 'C' having molecular formula C6H7N. The structure of 'A' is
View SolutionAn aromatic compound 'A' having molecular formula C7H6O2 on treating with aqueous ammonia and heating forms compound 'B'. The compound 'B' on reaction with molecular bromine and potassium hydroxide provides compound 'C' having molecular formula C6H7N. The structure of 'A' is
View SolutionAn aromatic compound A of the molecular formula C8H10O on reaction with iodine and dilute NaOH gives a yellow precipitate. The structure of the compound is expected to be :
View SolutionTwo compounds A and B with same molecular formula (C3H6O) undergo Grignard’s reaction with methylmagnesium bromide to give products C and D. Products C and D show following chemical tests.
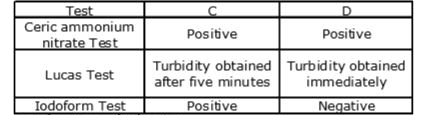
View SolutionAn aromatic compound 'A' having molecular formula C7H6O2 on treating with aqueous ammonia and heating forms compounds 'B'. The compound B on reaction with molecular bromine and potassium hydroxide provides compound 'C' having molecular formula C6H7N. The structure of 'A' is :
View Solution