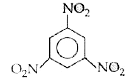
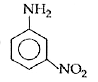
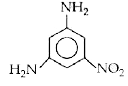
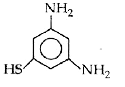
The major product (70% to 80%) of the reaction between m-dinitrobenzene with (NH4)2S is
A
B
C
D
Text Solution
The correct Answer is:B
(NH4)2S brings a selective reduction of one of the two −NO2 groups at m-position.
|
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:MTG GUIDEChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:CHECK YOUR NEET
Explore 25 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:MTG GUIDEChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:AIPMT/NEET MCQs
Explore 21 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:MTG GUIDEChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:AIPMT/NEET MCQs
Explore 21 Videos
- HALOALKANES AND HALOARENES Book:MTG GUIDEChapter:HALOALKANES AND HALOARENES Exercise:AIMPT/NEET
Explore 10 Videos
- POLYMERSBook:MTG GUIDEChapter:POLYMERSExercise:AIPMT/NEET
Explore 15 Videos
Similar Questions
Knowledge Check
The major product (70% to 80) of the reaction between m−dinitrobenzene with (NH4)2Sx is
A B
B C
C D
D
The mojor product (70% to 80%) of the reaction between m-dinitrobenzene with NH4HS is
A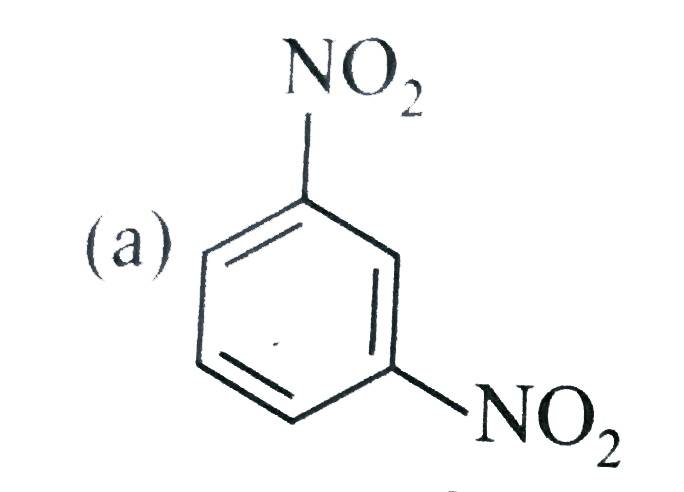 B
B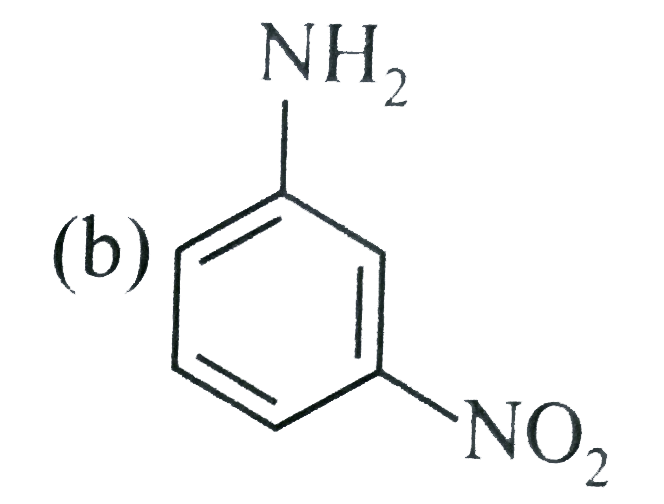 C
C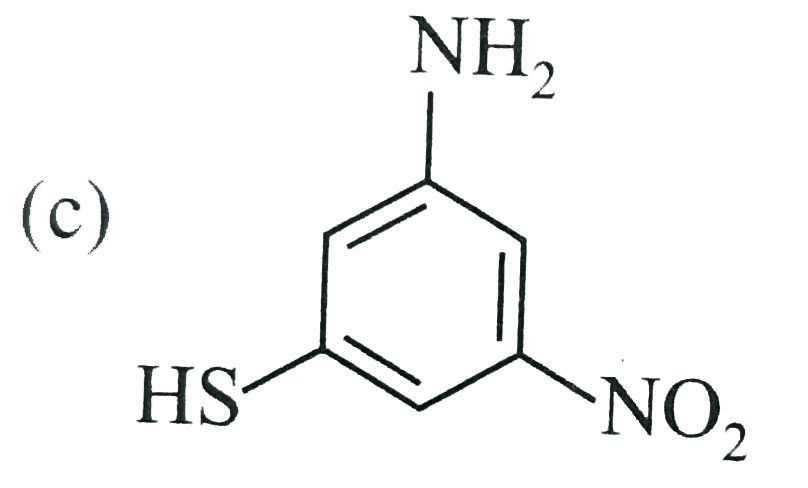 D
D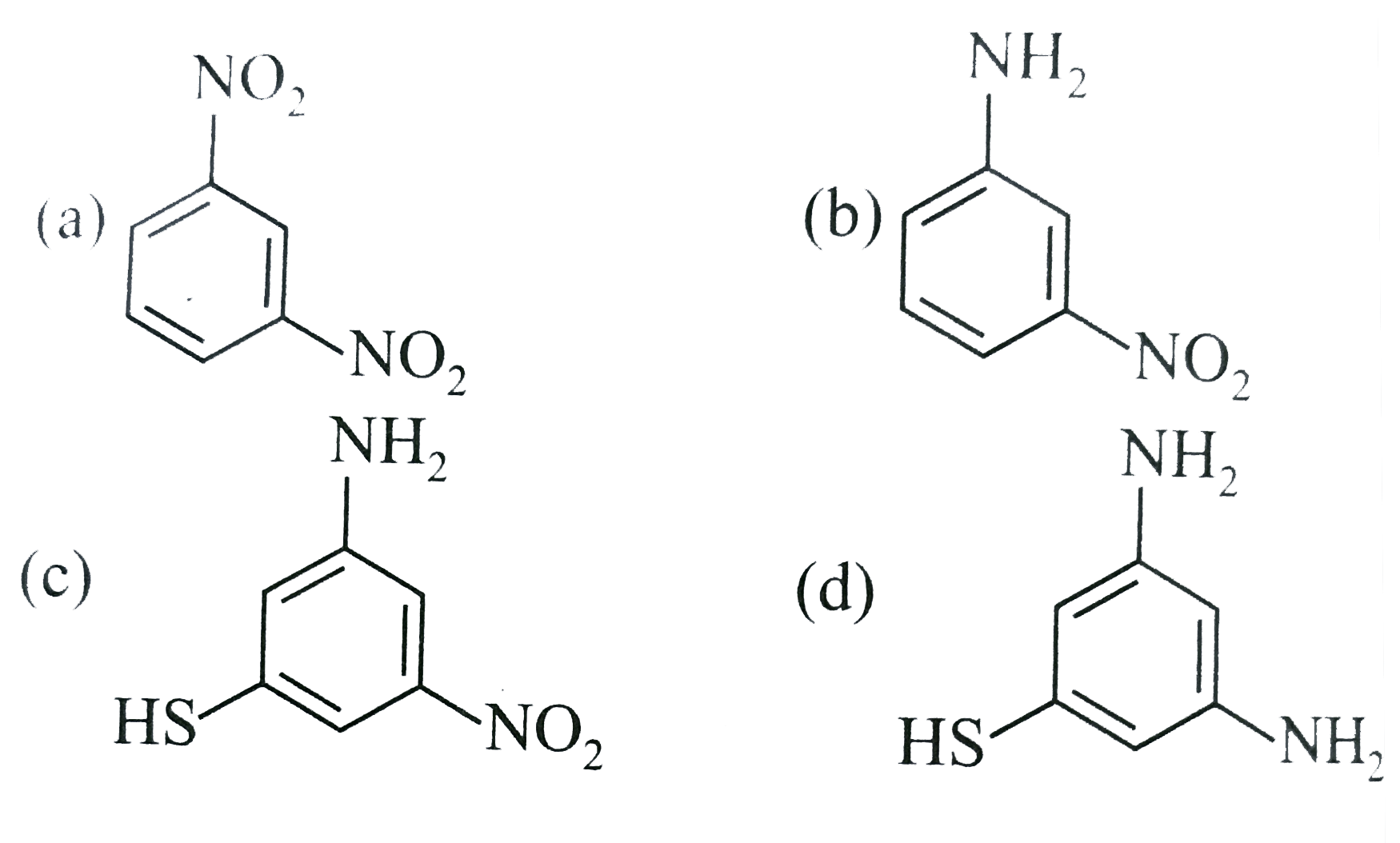
Which of the following is formed as a product when m -dinitrobenzene is treated with (NH4)2S ?
A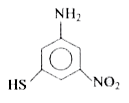 B
B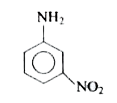 C
C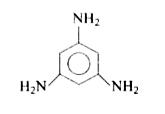 D
D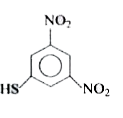
If first order reaction 80% reaction complete in 60 minute, What is t12 of reaction
View SolutionWhich of the following is foomed as a product when m-dinitrobenzene is treated with (NH4)2S ?
View SolutionAbout 70% of world livestock production is in
View Solutiont first order reaction 80% reaction complete in 60 minute, What is t12 of reaction
View SolutionThe major product of the following reaction is
(##MOD_SPJ_CHE_XII_P2_C12_E07_108_Q01.png" width="80%">
View Solution

