The two forms of D-glucopyranose obtained from the solution of D-glucose are called
isomer
anomer
epimer
enantiomer.
The correct Answer is:B
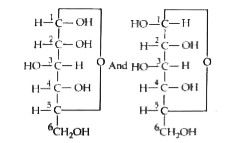
The configuration differe only at carbon no .1.
Topper's Solved these Questions
- BIOMOLECULESBook:MTG GUIDEChapter:BIOMOLECULESExercise:PROTEINS AND ENZYMES
Explore 57 Videos
- BIOMOLECULESBook:MTG GUIDEChapter:BIOMOLECULESExercise:HORMONES AND VITAMINS
Explore 25 Videos
- BIOMOLECULESBook:MTG GUIDEChapter:BIOMOLECULESExercise:AIPMT/NEET (MCQS)
Explore 19 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:MTG GUIDEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:AIPMT/NEET
Explore 21 Videos
- CHEMICAL KINETICSBook:MTG GUIDEChapter:CHEMICAL KINETICSExercise:AIPMT/NEET (MCQs)
Explore 22 Videos
Similar Questions
Knowledge Check
The two forms of D-glucopyranose obtained from solution of D-glucose are known as:
AanomersBepimersCenantiomersDgeometrical isomersTwo forms of D-glucopyranose, are called
AenantiomersBanomersCepimersDdiastereomersThe two structures of d-glucopyranose forms are
AanomersBenantiomersCepimersDdiastereoisomers
The two structures of D-glucopyranose forms are
View SolutionWhen either form of D-Glucose is dissolved In water, the solution gradually changes its optical rotation and finally attains a constant optical rotation of +52∘.
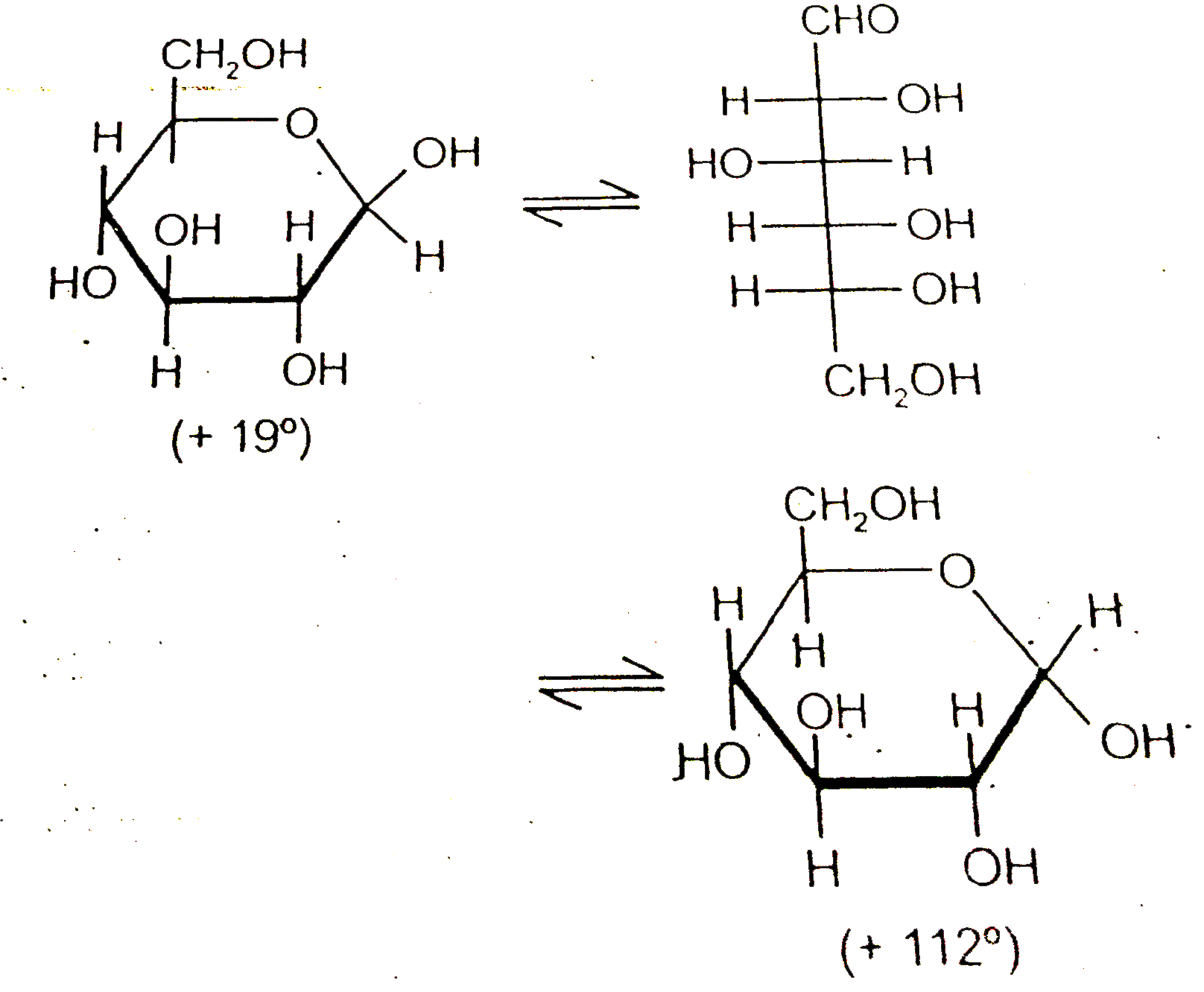
What is the percentage of open chain D-Glucose in solution?
View SolutionThe ΔH∘for the mutarotation of glucose in aqueous solution ,
α-D- glucose (aq)→β-D-glucose (aq)
has been measured in a microcalorimeter and found to be -1.16 kj .mol−1 . The enthalpies of solution of the two forms of glucose have been determined to be
α-D- glucose (aq)→α-D-glucose (aq)
ΔH∘=10.72KJmol−1
β-D- glucose (aq)→β−D-glucose (aq)
ΔH∘=4.68KJmol−1
Calculate ΔH∘(in KJ/mol) for the mutarotation of soild α-D-glucose→soild beta−D−glucose:
View Solutionβ−D−(+) Glucopyranose is
View SolutionThe aqueous solution of D-glucose contains two forms of D-glucopyranose, which are :
View Solution

