The bond length between hybridised carbon atom and other carbon atom is minimum in
A
propene
B
propyne
C
propane
D
butane
Text Solution
The correct Answer is:B
The C-C bond length = 1.54 Å, C=C bond length - 1.34 Å and C≡C bond length = 1.20 Å.
Since propyne has a triple bond, therefore it has minimum bond length.
|
Similar Questions
Knowledge Check
In which of the following compounds, the bond length between hybridised carbon atom and other carbon atom is minimum?
AButaneBPropyneCPropeneDButeneIn which of the following compounds, the bond length between hybridised carbon atom and other carbon atom is minimum?
AButaneBPropyneCPropeneDButaneThe bond length between sp3 hybridised carbon atom and other carbon atom is minimum in
APropaneBButaneCPropeneDPropyne
Carbon forms bonds with other atoms
View SolutionThe bond between carbon atom (1) and carbon atom (2) in compound
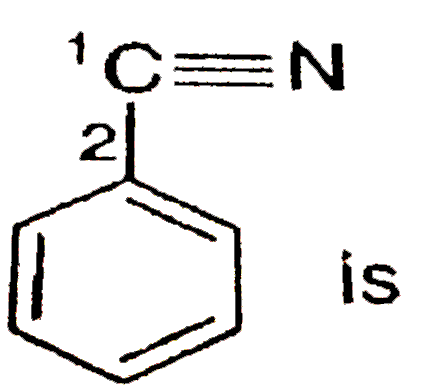
View SolutionThe bond between carbon atom 1 and carbon atom 2 in a compound N≡C−CH=CH−CH3 involved the hybrids as :
View SolutionThe double bond between the two carbon atoms in ethylene consists of
View SolutionThe bond between carbon atom (1) and carbon atom (2) in compound N≡C1−CH2=CH2, involves the hybridization as
View Solution

