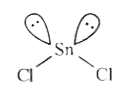
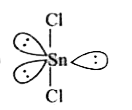
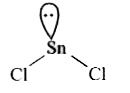
The atomic number of Sn is 50. The shape of gaseous SnCl2 molecule is
A
Cl-Sn-Cl
B
C
D
Text Solution
The correct Answer is:D
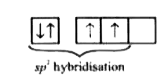
In SnCI2, Sn is sp2 hybridized. As such it has two bond pairs and one lone pair of electrons and hence structure (d) is correct.
|
Similar Questions
Atomic number of the centrtal atom in MCl_2 is 50. The shape of gaseous MCl_2 is given as
View SolutionAtomic number of the central atom in MCl2 is 50. The shape of gaseous MCl2 is given as
View Solutionआयनिक आकारों का सही क्रम है (परमाणु क्रमांक Ce=58, Sn=50, Yb=70 और Lu=71)
View SolutionThe number of collisions in gaseous molecules depends on ___________.
View SolutionThe shape of gaseous SnCl_2 is
View SolutionA piece of Sn foil is added to SnCl_2 solution for preserving it. Explain.
View SolutionName a gaseous element whose molecule and atom are identical.
View SolutionWhat is the shape of SO_3 molecule in gaseous state?
View Solution

