Which of the following is correct for the given equilibrium?
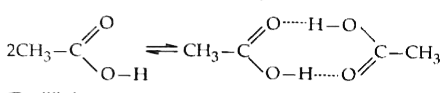
(Equilibrium constant for the reaction in water and benzene are KwaterandKbenzene respectively)
A
Kwater>Kbenzene
B
Kwater<Kbenzene
C
Kwater=Kbenzene
D
None of these
लिखित उत्तर
The correct Answer is:B
In benzene, acetic acid undergoes dimensions while in water, acetic acid remains in monomeric state, so
Kbenzne>Kwater
|
समान प्रश्न
ज्ञान जांच
The equilibrium constant (K) of a reaction may be written as
AK=e−ΔG/RTBK=e−ΔG∘/RTCK=e−ΔH/RTDK=e−ΔH∘/RTThe equilibrium constant (K) of a reaction may be written as
AK=e−ΔG/RTBK=e−ΔG∘RTCK=e−ΔH/RTDK=e−ΔH∘RTThe unit of equilibrium constant Kc for the reaction A+B⇔C would be
Amol−1LBmolL−1CmolLDNo unit
If degree of dissociation is 0.5 at equilibrium, then the equilibrium constant Kc for the given reaction is 2HI
View SolutionWhich is not correct relationship between ΔGΘ and equilibrium constant KP.
View SolutionWhich is the correct relationship between ΔG∘ and equilibrium constant Kp?
View SolutionWhich is the correct relationship between ΔG∘ and equilibrium constant Kp?
View Solution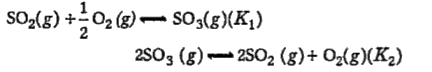
The correct relationship between equilibrium constantsK1 and K2 is
View Solution

