a. Write a electron dot structure for calcium and sulphur.
b. Show the formation of CaS by tranfer of electrons.
c. Name the ions present in this compound CaS. (At no Ca=20, S=16)Solution in Kannada
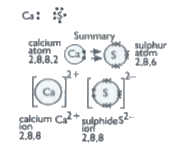
c. Calcium ion and sulphide ion.
Topper's Solved these Questions
- METALS AND NON METALSBook:ZEN PUBLICATIONChapter:METALS AND NON METALSExercise:ZEN ADDITIONAL QUESTIONS SECTION (LONG ANSWER [LA] TYPE 2 QUESTIONS)
Explore 14 Videos
- METALS AND NON METALSBook:ZEN PUBLICATIONChapter:METALS AND NON METALSExercise:ZEN ADDITIONAL QUESTIONS SECTION (SHORT ANSWER [SA] TYPE 1 QUESTIONS)
Explore 28 Videos
- CHEMICAL REACTIONS AND EQUATIONSBook:ZEN PUBLICATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:ZEN ADDITIONAL QUESTIONS SECTION (LONG - ANSWER [LA]TYPE QUESTIONS )
Explore 27 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBook:ZEN PUBLICATIONChapter:PERIODIC CLASSIFICATION OF ELEMENTSExercise:QUESTIONS
Explore 2 Videos
Similar Questions
State one property of each of the following which makes them suitable for the followng:
a. Copper and aluminium for making electric wires
b. Gold, platinium and silver are used to make jewellery
c. Copper to make hiot water tank
d. Graphite in making electrodes
e. Alloys for making bells and strings in musical instruments.
View SolutionYou are given three metals: sodium, magnesium, and copper. Suggest any two activities to arrange them in order of their reactivity.
View SolutionBalance the following chemical equation
P4+O2→P2O5
View SolutionThe electronic configuration , group number and period number of an element with atomic number 10 is
View SolutionThe electronic configuration of calcium ion (Ca2+) is
View Solution(a) Write the electron dot structure for calcium and oxygen. The atomic numbers of calcium and oxygen are 20 and 8 respectively. (b) Show the formation of calcium oxide by the transfer of electrons. (c) Ionic compounds are high melting solids. Give reason.
View Solutionइन के इलेक्ट्रॉनिक विन्यास लिखें।
Ca(20)
View SolutionThe electronic configuration of calcium ion (Ca2+) is :-
View Solutiona. Write electron dot diagram for chlorine (At No. 17) and Calcium (At No 20) show the formation of Calcium chloride by transfer of electrons.
View Solutiona. Explain the formation of Calcium Chloride with the help of electron dot structure. (At numbers: Ca=20,Cl=17)
b. Why do ionic compounds not conduct electricity in solid state but conduct electricity in molten and aqueous state?
View SolutionThe electronic configuration for Ca2+ ion is :
View Solutionकैल्शियम (Ca2+) आयन का इलेक्ट्रॉनिक विन्यास है
View Solution(i) Write the electron-dot structures for sodium, oxygen and magnesium. (ii) Show the formation ofNa2O and MgO by the transfer of electrons. (iii) What are the ions present in these compounds ?
View SolutionWrite electron dot structure (Lewis structure) of Na, Ca, B, Br, Xe, As, Ge, N3−.
View Solution(a) Write the electron dot structure for calcium and oxygen. The atomic numbers of calcium and oxygen are 20 and 8 respectively.
(b) Show the formation of calcium oxide by the transfer of electrons.
(c) Ionic compounds are high melting solids. Give reason.
View Solution