.3Li,14Si,2He,11Na,15P. Which of these elements belong to period 3?
Electronic configuration :
.3Li−2,1.14Si−2,8,4.2He−2.11Na−2,8,1.15P−2,8,5
Elements .14Si,11Naand.15P belongs to period 3.
Reason : Elements with same number of shells occupied by electrons belong to the same period. Here, Si , Na and P have three shells, hence, all these elements belong to third peroid.
Similar Questions
Knowledge Check
There are 14 elements in actinoid series. Which of the following elements does not belong to this series?
AUBNpCTmDFmThere are 14 elements in actinoid series. Which of the following elements does not belong to this series?
AUBNpCTmDFm
.13Al,14Si,11Na,12Mg,16S Which of the above elements has the highest metallic character ?
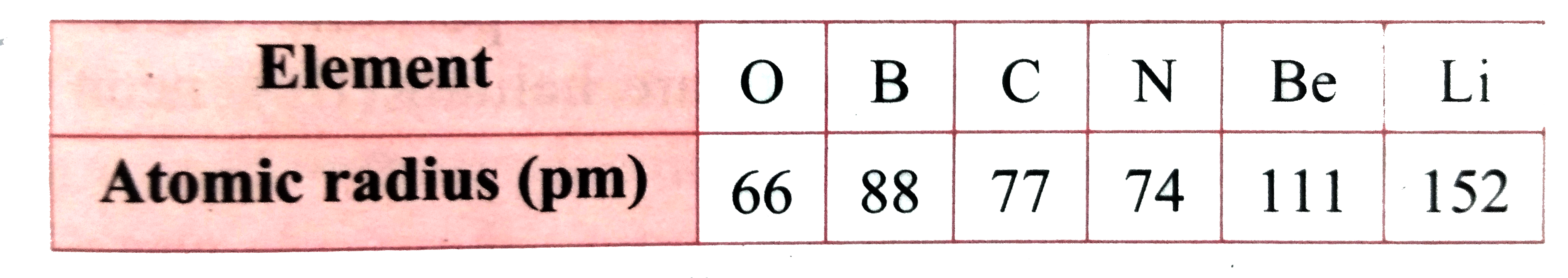
View Solution.19K,3Li,11Na,4Be. Which of these atoms have smallest atomic radius?
View Solution.11Na,15P,17Cl,14Si,12Mg. Which of these has largest atoms ?
View SolutionConsider the following elements:
Na,Ca,Al,K,Mg,Li
(a) Which of these elements belong to the same period of the periodic table?
(b) Which of these elements belong to the same group of the periodic table?
View SolutionState the period to which the above elements belong.
View SolutionThe elements of a period of the periodic table are given below in order from left to right with one of its element missing:
Na Mg Al Si S Cl Ar .
(a) To which period do these elements belong? (b) One element of this period is missing. Which is the missing element and where should it be placed? (c) Which one of these elements in this period shows the property of catenation? (d) Which one of these elements is least reactive? (e) Identify the halogen from this period.
View Solution
