Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers.
K[Cr(H2O)2(C2O4)2]
K[Cr(H2O)2(C2O4)2)orK[Cr(H2O)2(ox)2](where, ox= oxalate ion
(a) It exists as geometrical isomers, cis and transforms.
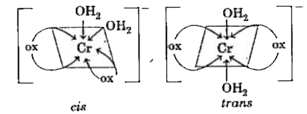 (In cis-form, the same groups occupy adjacent positions, while in trans-form, they are present at alternate positions.)
(In cis-form, the same groups occupy adjacent positions, while in trans-form, they are present at alternate positions.)
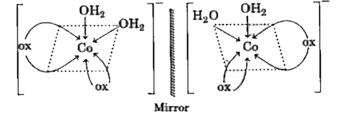
(b) The cis-isomer can also exist as a pair of optical isomers (i.e. d-and l-forms) (due to the absence of plane of symmetry).
समान प्रश्न
Indicate the types of isomerism exhibited by the following complexes and draw the structures of these isomers K[Cr(H_2O)_2(C_2O_4)_2]
View SolutionIndicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: K[Cr(H_2O)_2(C_2O_4)_2]
View SolutionIndicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [CO(NH3)5(NO2)](NO3)2
View SolutionIndicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [Pt(NH_3)(H_2O)CI_2]
View SolutionIndicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers.
[Co(NH3)5(NO2)](NO3)2
View SolutionIndicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers. [Pt(NH_3)(H_2O)Cl_2]
View SolutionIndicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers. [Cr(H_(2)O)_(5)(SCN)]^(2+)
View Solution

