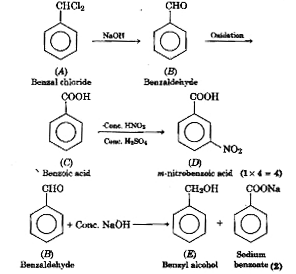
AN organic compound A(C7H6Cl2) on treatment with NaOH solution gives another compound B (C7H6O) . B on oxidation gives an acid (C7H5O2) which on treatment with a mixture of cone. HNO3andH2SO4 gives a compound D (C7H5NO2) B on treatment with conc. NaOH gives compound E(C7H8O)andC6H5COONa. Deduce the structures of A,B,C,D and E.
Since eompound A on treatment with NaOH gives C7H6O, i.e. only one O-atom is introduced, both the Cl atoms must be present on the same carbon atom The molecular formula C7H6O suggest the structure of B is C5CHO. Thus A must be C6H6CHCl2. The relevent equations are :
समान प्रश्न
An organic compound A of molecular formula C6H6O gives violet colouration with neutral FeCl3. Compound A on treatment with metallic Na gives compound B. Compound B on treatment with CO2 at 400 K under pressure gives C. This product on acidification gives compound D (C7H6O3) which is used in medicine. Identify A,B, C and D and explain the reaction.
View SolutionCompound A of molecular formula C_(2)H_(8) is treated with chlorine and then with NaOH to get compound B of molecular formula. C_(2)H_(8)O . B on oxidation by acidified K_(2)Cr_(2)O_(7) gives compound C of molecular formula C_(7)H_(6)O . Compound C on treatment with 50% caustic soda gives the compound B and also D. Find A,B,C and D. Explain the reactions.
View SolutionAn organic compound of molecula formula C6H5ONa is heated with CO2 at 400 K gives compound (A) of molecular formula C7H5O3Na. Compound (A) on treating with HCl gives (B). B on further reactions wiith NaOH / CaO gives compound (C ) of molecular formula C6H6O which on treatment with nitrous acid at 200 K gives compound (D ). Identify (A), (B) , ( C) and (D) are explain the reactions.
View SolutionC_(12) H _(12) O _(11) overset(H_(2) SO _(4)) to H _(2) O + A overset( H _(2) SO _(4))to B + C + H _(2)O If the compound C is an oxide of group VIA elemet then the compound B is
View SolutionAn organic compound containing C, H, N & O (A) on treatment with NaOH & Br_2 from (B) B on treatment with NaNO_2 and Hydrochloric acid forms C a primary alcohol wihc on iodoform test gives yellow-ish crystalline precipitate identify A, B & C. Give reasons and chemi-cal reaction.
View SolutionThe aqueous solution of a nitrogen and chlorine containing organic compound (A) is acidic to litmus. (A) on treatment with aqueous NaOH, it gives a compound (B) containing nitrogen, but not chlorine. Compound (B) on treatment with C_6 H_5SO_2 Cl in the presence of NaOH gives an insoluble product (C) C13H13NO2 S. Give the structures of (A) and (B).
View Solution(i) A powdered substance (A) on treatment with fusion mixture gives a green coloured compound (B). (ii) The solution of (B) in boiling water on acidification with dilute H2SO4 gives a pink coloured compound (C).
(iii) The aqueous solution of (A) on treatment with NaOH and Br2− water gives a compound (D).
(iv) A solution of (D) in conc. HNO3 on treatment with lead peroxide at boiling temperature produced a compound (E) which was of the same colour at that of (C) . Identify A,B,C,D,E
View SolutionEthanoic acid on heating with ammonia forms compound A which on treatment with bromine and sodium hydroxide gives compound B. Compound B on treatment with NaNO2/dil. HCl gives compound C. The compounds A, B and C are
View Solution

