Consider the bromination of butane using sufficient bromine to cause dibromination. After these reaction is over, you separate all the dibromobutane isomers by gas chromatography or by fractional distillation. How many fractions would you obtain, and what compounds would the individual fractions contain? Which if any of the fractions would be optically active?
The construction of handheld models will help in solving this problem. First, decide how many constitutional isomers are possible by replacing two hydrogens of butane with two bromine atoms. There are six: 1, 1-dibromobutane, 1, 2-dibromobutane, 2, 2-dibromobutane, 2, 3-dibromobutane, 1, 3-dibromobutane, and 1, 4-dibromobutane. Then recall that constitutional isomers have different physical properties (i.e., boiling points and retention times in a gas chromatograph), so there should be at least six fractions. In actuality there are seven. See fractions (a)-(g) below. We soon see why there are seven fractions if we examine each constitutional isomer looking for chirality centers and stereoisomers. Isomers (a), (c ) and (g) have no chirality centers and are, therefore, achiral and are optically inactive. 1, 2-Dibromobutane in fraction (b) and 1, 4-dibromobutane in fraction (f ) each have one chirality center and, because there is no chiral influence on the reaction, they will be formed as a 50:50 mixture of enantiomers (a racemate). A racemate cannot be separated by distillation or conventional gas chromatography, there fore, fractions (b) and (f ) will not be optically active. 2, 3-Dibromobutane has two chirality centers and will be formed as a racemate [fraction (d)] and as a meso compound, fraction (e ). Both fractions will be optically inactive. The meso compound is a diastereomer of the enantiomers in fraction (d) (and has different physical properties from them), therefore, it is separated from them by distillation or gas chromatography.

Topper's Solved these Questions
- RADICAL REACTIONSBOOK - MS CHOUHANCHAPTER - RADICAL REACTIONSEXERCISE - Practice Problem
2 Videos
- RADICAL REACTIONSBOOK - MS CHOUHANCHAPTER - RADICAL REACTIONSEXERCISE - Additional Objective Questions (Single Correct Choice Type)
22 Videos
- PRACTICAL ORGANIC CHEMISTRYBOOK - MS CHOUHANCHAPTER - PRACTICAL ORGANIC CHEMISTRYEXERCISE - Level 2
5 Videos
- REACTION OF AROMATIC COMPOUNDS BOOK - MS CHOUHANCHAPTER - REACTION OF AROMATIC COMPOUNDS EXERCISE - ADDITIONAL OBJECTIVE QUESTIONS (MULTIPLE CORRECT CHOICE TYPE)
2 Videos
Similar Questions
Benzylic radicals, due to the adjacent benzene ring, have even greater possibility for delocalization than allylic radicals. Draw contributing resonance structures that show this delocalization for the benzylic radical derived from methylbenzene.
View SolutionA mixture of ethyl iodide and n-propyl iodide is subjected to Wurtz reaction. The hydrocarbon that will not be formed is
View SolutionGiven are the isomers of C8H8O2.
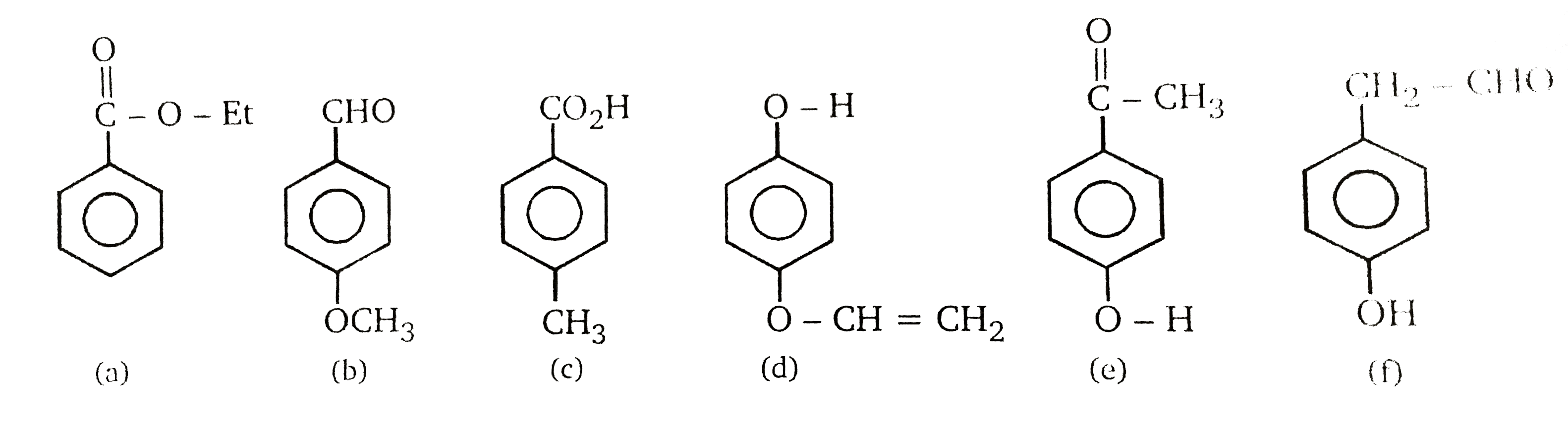
Which isomer gives positive iodoform test?
View SolutionToluene, when treated with Br2/Fe , gives p-bromotoluene as the major product, because the CH3 group
View SolutionThere are four dimethylcyclopropane isomers.
a. Write the three-dimensional formulas for these isomers.
b. Which of these isomers is chiral?
c. If a mixtrue consisting of 1mol of each of these isomers is subjected to simple gas chromatography, how many fractions would be obtained and how many compounds each fraction will have?
d. How many of these fractions would be optically active?
View SolutionTake a living tissue, grind it in trichloroacetic acid using pestle and mortal, and then strain it, you would obtain two fractions : acid-soluble and acid-insoluble fraction. Acid-insoluble fraction does not contain
View SolutionHow many fractions can be obtained after fractional distillation of all dibromo derivatives of n-butane?
View SolutionHow many fractions will be obtained on fractional distillation of mixture of all dimethyl cyclopentance?
View SolutionHow many total products will be obtained by monochlorination of 2- methyl butane and how many can be separated by fractional distillation?
View SolutionWhich of the following pair of isomers can not be separated by fractional crystallisation or fractional distillation:
View SolutionWhich of the following pair of isomers cannot be separated by fractional crystallization or distillation or chromatography ?
View SolutionFrom the methods (or techniques) of distillation, filtration, fractional distillation, chromatography, crystallisation, select and write down the technique you would use to separate :Constituents of colouring matter in ink.
View SolutionFrom the methods (or techniques) of distillation, filtration, fractional distillation, chromatography, crystallisation, select and write down the technique you would use to separate : Hydrated copper (II) sulphate or blue vitriol from its aqueous solution.
View SolutionFrom the methods (or techniques) of distillation, filtration, fractional distillation, chromatography, crystallisation, select and write down the technique you would use to separate : Nitrogen from liquid air.
View SolutionFrom the methods (or techniques) of distillation, filtration, fractional distillation, chromatography, crystallisation, select and write down the technique you would use to separate : Unused zinc after the reaction of excess of zinc with dilute sulphuric acid.
View Solution

