Choose the incorrect statement about the following two reactions
(i) CH3−H∣C∣H−H+⋅Cl→CH3−H∣C∣H⋅+H−Cl ΔH∘=−2 kcal/mol
(ii) CH3−H∣C∣H−H+⋅Br→CH3−H∣C∣H⋅+H−Br ΔH∘=+13 kcal/mol
Reaction (i) would be expected to be faster than reaction (ii)
According to Hammound's postulate the transition state of reaction (ii) resembles the product
According to Hammound's postulate the transition state of reaction (i) resembles the reactant
The C−H bond of reaction (ii) is completely broken in the transition state.
The correct Answer is:D
Topper's Solved these Questions
- RADICAL REACTIONSBook:MS CHOUHANChapter:RADICAL REACTIONSExercise:Additional Objective Questions (Integer Type)
Explore 4 Videos
- RADICAL REACTIONSBook:MS CHOUHANChapter:RADICAL REACTIONSExercise:Practice Problem
Explore 2 Videos
- PRACTICAL ORGANIC CHEMISTRYBook:MS CHOUHANChapter:PRACTICAL ORGANIC CHEMISTRYExercise:Level 2
Explore 5 Videos
- REACTION OF AROMATIC COMPOUNDS Book:MS CHOUHANChapter:REACTION OF AROMATIC COMPOUNDS Exercise:ADDITIONAL OBJECTIVE QUESTIONS (MULTIPLE CORRECT CHOICE TYPE)
Explore 2 Videos
Similar Questions
How many chiral compounds are possible on monochlorination of 2-methyl butane?
View SolutionBenzylic radicals, due to the adjacent benzene ring, have even greater possibility for delocalization than allylic radicals. Draw contributing resonance structures that show this delocalization for the benzylic radical derived from methylbenzene.
View SolutionGiven are the isomers of C8H8O2.
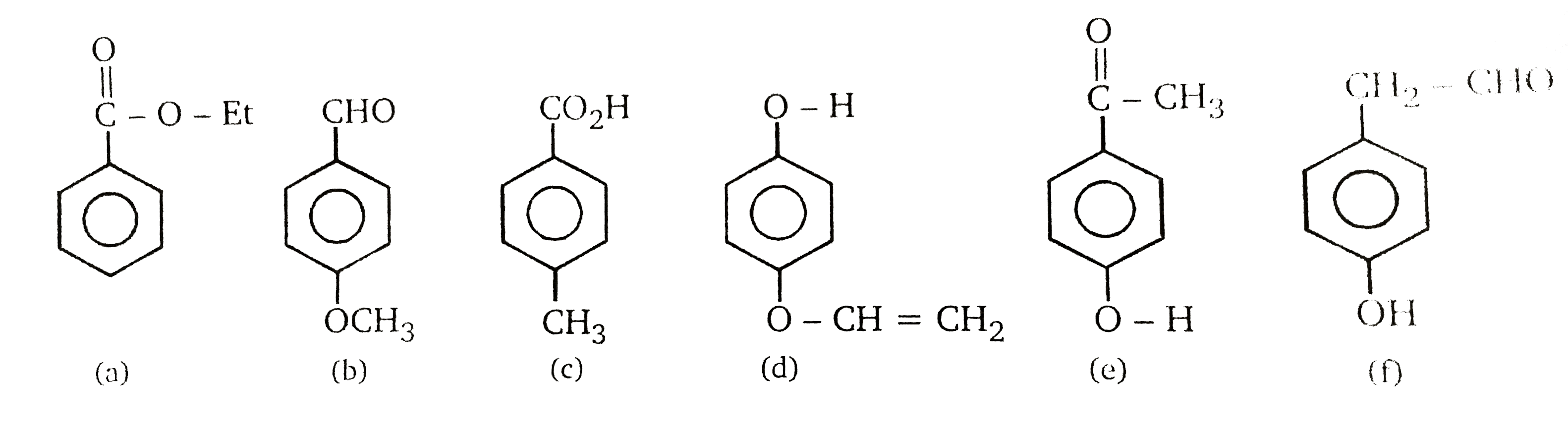
Which isomer gives positive iodoform test?
View SolutionToluene, when treated with Br2/Fe , gives p-bromotoluene as the major product, because the CH3 group
View SolutionHow would you name the following compounds?
(i) CH3−CH2−Br , (ii) H−H∣C=O
(iii) H−H∣C∣H−H∣C∣H−H∣C∣H−H∣C∣H−C≡C−H
View SolutionWrite the names of the following compounds. (a) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-overset(O)overset(||)C-OH , (b) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-C-=C-H (c ) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-overset(H)overset(|)C=O , (d) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-OH
View SolutionIdentify and name the functional groups present in the following compounds. (a) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-OH , (b) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-overset(O)overset(||)C-OH (c ) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-overset(O)overset(||)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-H , (d) H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-overset(H)overset(|)C=overset(H)overset(|)C-H
View SolutionIdentify the name of the functional group in the following compounds:
(i) H−H∣C∣H−H∣C∣H−NH2
(ii) H−H∣C∣H−H∣C∣H−Br
(iii) H−H∣C∣H−O∣∣C−H
(iv) H−H∣C∣H−O∣∣C−H∣C∣H−H.
View Solutionनिम्न यौगिकों का नामकरण कैसे करेंगे ?
(i) CH3−CH2−Br
(ii) H−H∣C=O
(iii) H−H∣C∣H−H∣C∣H−H∣C∣H−H∣C∣H−C≡C−H
View Solutionनिम्न यौगिकों का नामकरण कैसे करेंगे।
(i)CH3CH2−Br
(ii)H−H∣C=O
(iii)H−H∣C∣H−H∣C∣H−H∣C∣H−H∣C∣H−C≡C−H
View SolutionHow would you name the following compounds ?
(i)CH3−CH2−Br (ii)H−CH=O
(iii)H−C=C−H∣C∣H−H∣c∣H−H∣c∣H−H∣C∣H−H
View SolutionThe IUPAC name of the compound shown below is: H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(Cl)overset(|)C-Cl
View SolutionThe IUPAC name of the compound shown below is:
H−H∣C∣H−H∣C∣H−Cl∣C∣H−Cl
View Solution(a ) निम्नलिखित यौगिकों का नामांकन कैसे करेंगे?
(i) CH3−CH2−Br
(ii) H−−H∣C∣H=O
(iii) H−H∣C∣H−H∣C∣H−H∣C∣H−H∣C∣H−C≡C−H
View SolutionHow would you name the following compounds ?
(i) CH3−CH2−Br
(ii) H−H∣C=O
(iii) H−H∣C∣H−H∣C∣H−H∣C∣H−H∣C∣H−C≡C−H
View SolutionHow would you name the following compounds?
(i) CH3−CH2−Br , (ii) H−H∣C=O
(iii) H−H∣C∣H−H∣C∣H−H∣C∣H−H∣C∣H−C≡C−H
View Solution

