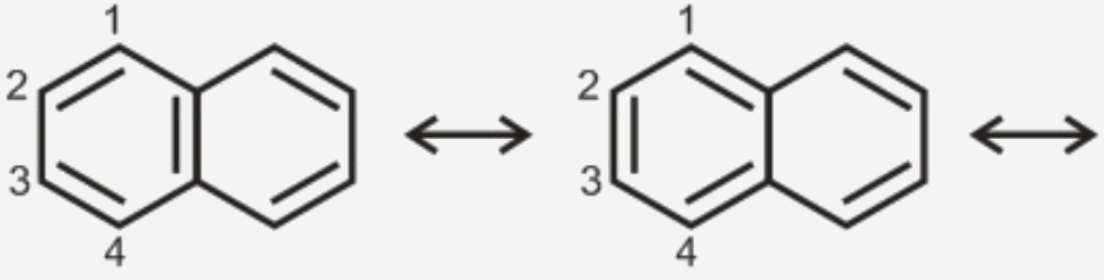
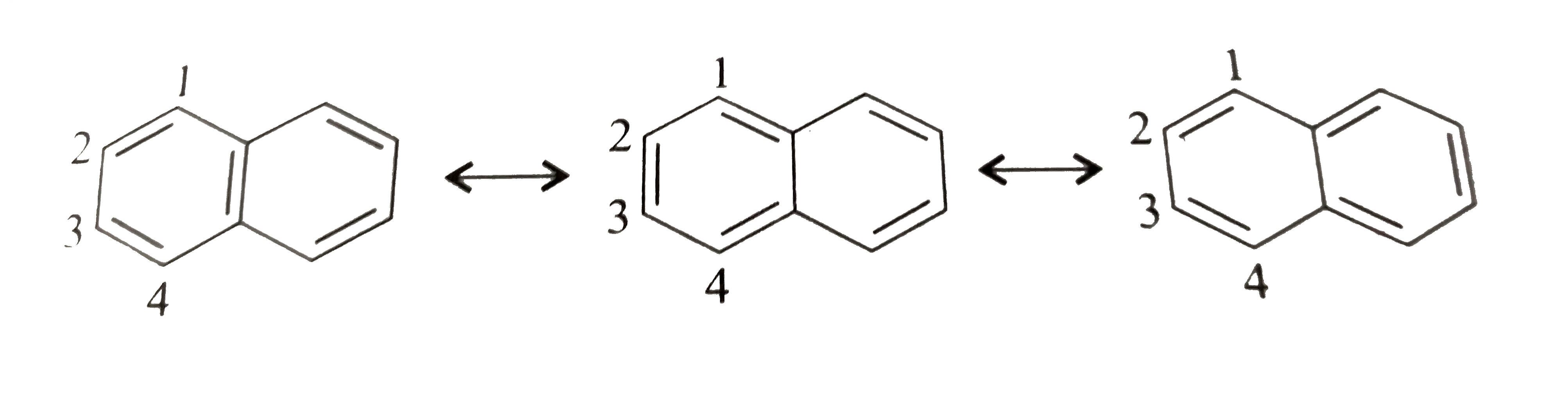
These are three cononical structures of napthalene. Examine them and find correct statement among the following :
A
All C-C bonds are os same length.
B
Cl-C2 bond is shorter than C2-C3 bond.
C
Cl-C2 bond is longer C2-C3 bond
D
None of these
Text Solution
The correct Answer is:B
|
Topper's Solved these Questions
- AROMATIC COMPOUNDSBOOK - MS CHOUHANCHAPTER - AROMATIC COMPOUNDSEXERCISE - Additional Objective Questions (Linked Comprehension Type)
6 Videos
- AROMATIC COMPOUNDSBOOK - MS CHOUHANCHAPTER - AROMATIC COMPOUNDSEXERCISE - Additional Objective Questions (Integer Type)
2 Videos
- AROMATIC COMPOUNDSBOOK - MS CHOUHANCHAPTER - AROMATIC COMPOUNDSEXERCISE - Solved Problem
48 Videos
- AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESBOOK - MS CHOUHANCHAPTER - AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESEXERCISE - ADDITIONAL QUESTION (SINGLE CORRECT CHOICE TYPE )
32 Videos
- BIOMOLECULESBOOK - MS CHOUHANCHAPTER - BIOMOLECULESEXERCISE - Level 2
1 Video
Similar Questions
Knowledge Check
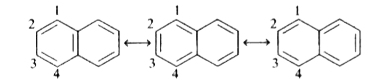
There are three canonical structures of napthalene. Examine them and find correct statement among the following:AAllC–C bonds are of some lengthBC1−C2 bond is shorter than C2−C3 bond.CC1−C2 bond is longer than C2−C3 bondDNone .There are three canonical structures of naphthalene. Examine them and find correct statement among the following :
 AAll C-C bonds are of same lengthBC1−C2 bond is shorter than C2−C3 bondCC1−C2 bond is larger than C2−C3 bondDNone of the above
AAll C-C bonds are of same lengthBC1−C2 bond is shorter than C2−C3 bondCC1−C2 bond is larger than C2−C3 bondDNone of the aboveThere are three canonical structures of naphtahlene. Examine them and find correct statement among the following :
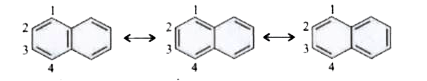 AAll C-C bonds are of same lengthBC1−C2 bond is shorter than C2−C3 bondCC1−C2 bond is larger than C2−C3 bondDNone of the above
AAll C-C bonds are of same lengthBC1−C2 bond is shorter than C2−C3 bondCC1−C2 bond is larger than C2−C3 bondDNone of the above