Designate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
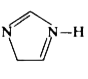
The compound is aromatic.
Topper's Solved these Questions
- AROMATIC COMPOUNDSBOOK - MS CHOUHANCHAPTER - AROMATIC COMPOUNDSEXERCISE - Additional Objective Questions (Single Correct Choice Type)
20 Videos
- AROMATIC COMPOUNDSBOOK - MS CHOUHANCHAPTER - AROMATIC COMPOUNDSEXERCISE - Additional Objective Questions (Linked Comprehension Type)
6 Videos
- AROMATIC COMPOUNDSBOOK - MS CHOUHANCHAPTER - AROMATIC COMPOUNDSEXERCISE - Subjective Problems
3 Videos
- AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESBOOK - MS CHOUHANCHAPTER - AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESEXERCISE - ADDITIONAL QUESTION (SINGLE CORRECT CHOICE TYPE )
32 Videos
- BIOMOLECULESBOOK - MS CHOUHANCHAPTER - BIOMOLECULESEXERCISE - Level 2
1 Video
Similar Questions
Which of the following compounds is likely to adopt a planar conformation ?
View SolutionWe have now learned that benzene is unsually stable, and that this stability seems to be correlated with the overlap of its carbon 2p orbitals to form π molecular orbitals. In 1931. Erich Huckel (1896 - 1980), a German chemist physicist, elucidated with molecular orbital arguments the criteria for this sort of stability, which has come to be called aromaticity.
Criteria for aromaticity :
* Aromatic compounds contain one or more rings that have a cyclic arragement of p-orbitals. Thus, aromaticity is a property of certain cyclic compounds.
* Every atom of an aromatic ring has a p-orbital.
* Aromatic rings are planar.
The cyclic arrangements of p-orbitals in an aromatic compound must contain 4n+2π electrons, where n is any positive integer (0, 1, 2, ....). In other words, an aromatic ring must contain 2, 6, 10 π electrons.
Which of the following compound is not anti-aromatic ?
View Solution
Double bond equivalent of D is:
View Solution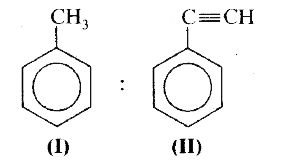
In compound (I) and (II) inductive effect of phenyl is
View SolutionHow many compound which is given below is isomer of D-Glucose ?
D-Mannose, D-Fructose, D-Gulose, D-Idose, D-Galactose, D-Arabinose, D-Ribose.
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
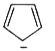
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.

View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
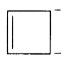
View SolutionDesignate whether each of the following compounds is aromatic, non-aromatic or anti-aromatic.
View SolutionClassify each as aromatic, anti-aromatic and non-aromatic compound.
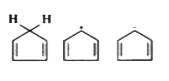
View Solution

