At the point of intersection of the two curves shown the concentration of B is given by _______for the first reaction A→nB.
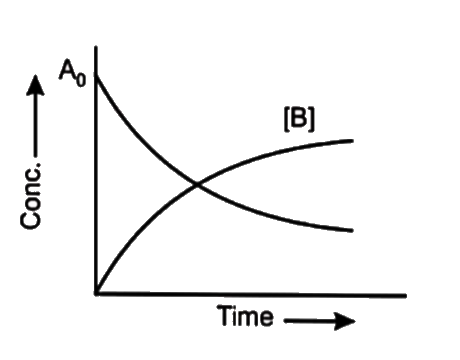
nA02
A0n−1
nA0n+1
(n−1n+1)A0
The correct Answer is:C
Step by step video solution for At the point of intersection of the two curves shown the concentration of B is given by _______for the first reaction A to nB. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
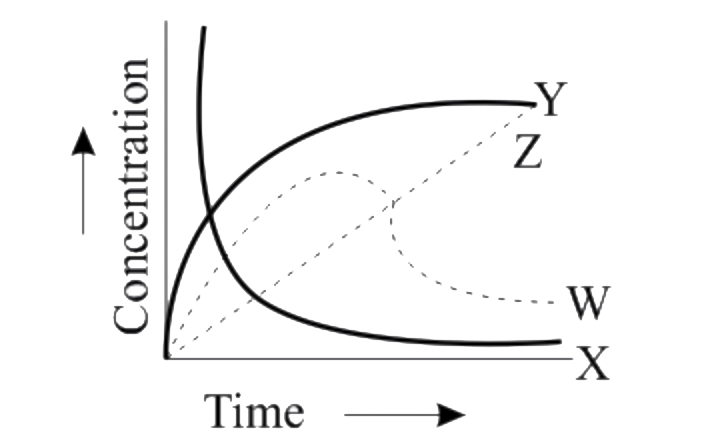
The accompanying figure depicts the change in concentration of species X and Y for the reaction XtoY , as a function of time. The point of intersection of the two curves represents:
View SolutionThe following graph X to For the Y reaction, the concentration of X and Y changes over time, showing the point of intersection of both curves -
View Solutionप्रथम कोटि की अभिक्रिया A→nB के लिए दिखाए गए दो वक्रों के प्रतिच्छेद बिंदु पर, B की सांद्रता ------ द्वारा दी जाती है :
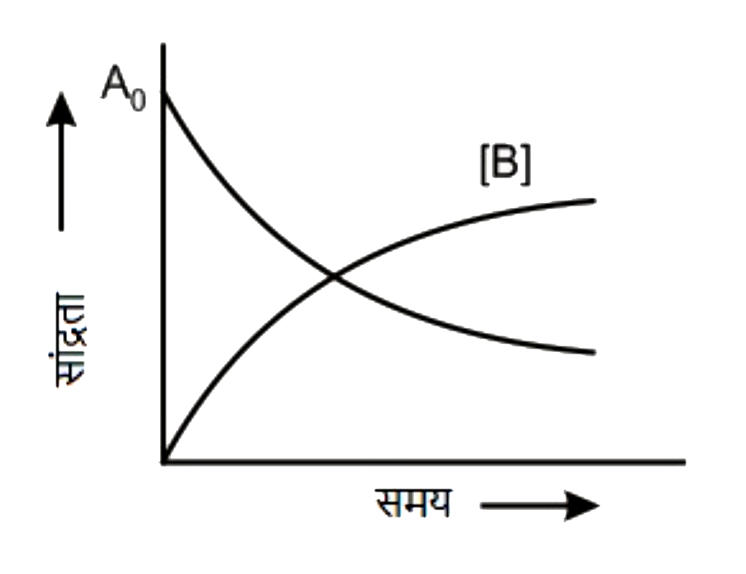
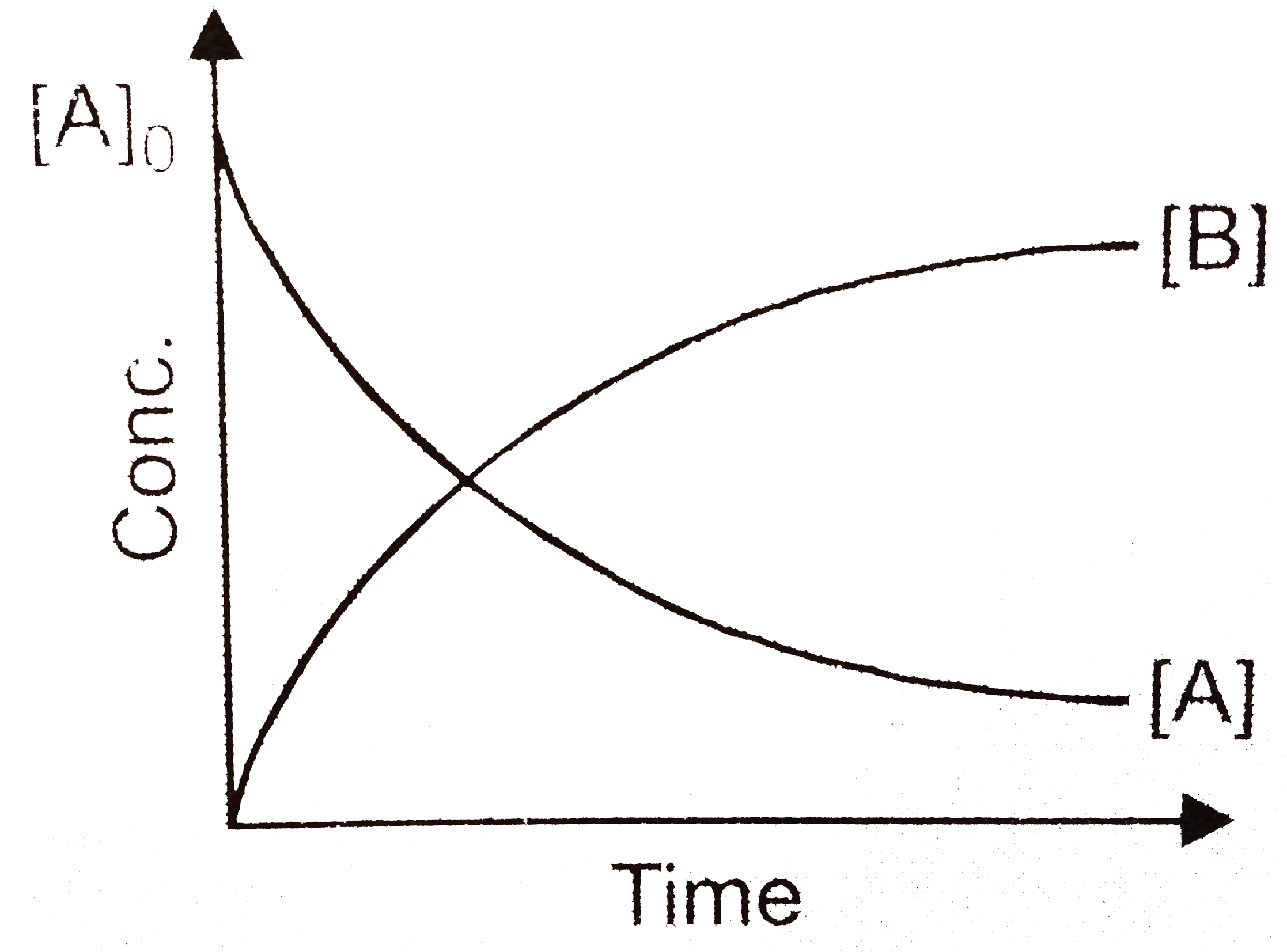
View SolutionAt the point of intersection of the two curves shown, the conc. of B is given by ……… for, A→nB:
View SolutionFor the reaction, A+B→C+D. The variation of the concentration of the products is given by the curve.
View SolutionIn the reaction A→ B , if the concentration of A is plotted against time, the curves obtained will be as shown in Fig. 1 and 2. Predict the order of the reactions.
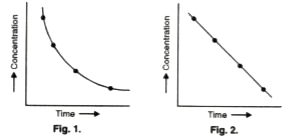
View SolutionIn the reaction A→ B , if the concentration of A is plotted against time, the curves obtained will be as shown in Fig. 1 and 2. Predict the order of the reactions.

View Solution

