In thermodynamic process, 200 Joules of heat is given to a gas and 100 Joules of work is also done on it. The change in internal energy of the gas is
100 J
300 J
419 J
24 J
The correct Answer is:B
To solve the problem, we will use the First Law of Thermodynamics, which states:
ΔU=Q−W
Where:
- ΔU is the change in internal energy,
- Q is the heat added to the system,
- W is the work done by the system.
1. Identify the Given Values:
- Heat given to the gas, Q=200Joules
- Work done on the gas, W=100Joules
2. Determine the Sign of Work:
- Since work is done on the gas, we consider it as negative in our equation:
W=−100Joules
3. Apply the First Law of Thermodynamics:
Substitute the values into the equation:
ΔU=Q−W
ΔU=200J−(−100J)
4. Simplify the Equation:
ΔU=200J+100J
ΔU=300Joules
5. Conclusion:
The change in internal energy of the gas is:
ΔU=300Joules
Final Answer:
The change in internal energy of the gas is 300 Joules.
---
- ΔU is the change in internal energy,
- Q is the heat added to the system,
- W is the work done by the system.
- Heat given to the gas, Q=200Joules
- Work done on the gas, W=100Joules
- Since work is done on the gas, we consider it as negative in our equation:
W=−100Joules
Substitute the values into the equation:
ΔU=Q−W
ΔU=200J−(−100J)
ΔU=200J+100J
ΔU=300Joules
The change in internal energy of the gas is:
ΔU=300Joules
The change in internal energy of the gas is 300 Joules.
समान प्रश्न
In a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy of the process?
View SolutionThe pressure of given mass of a gas in a thermodynamic system is changed in such a way that 20 joule of heat is released from the gas and 8 joule of work is done on the gas. If the initial internal energy of the gas was 30 joule then final internal energy will be
View Solution
ज्ञान जांच
A system is provided 50 J of heat and work done on the system is 10 J. The change in internal energy during the process is
A40 JB60 JC80 JD50 J.
In a process, 701J of heat is absorbed by a system and 394J of work is done by the system. What is the change in internal energy for the process?
View SolutionIn a thermodynamic process, pressure of a fixed mass of gas is changed in such manner that the gas releases 30 joule of heat and 18 joule of work was done on the gas. If the initial internal energy of the gas was 60 joule, then the final internal energy is 8x joule. find the value of x
View SolutionA system absorbs 1500 joule of energy as heat and produces 500 joule of work .the change in internal energy of the system will be :
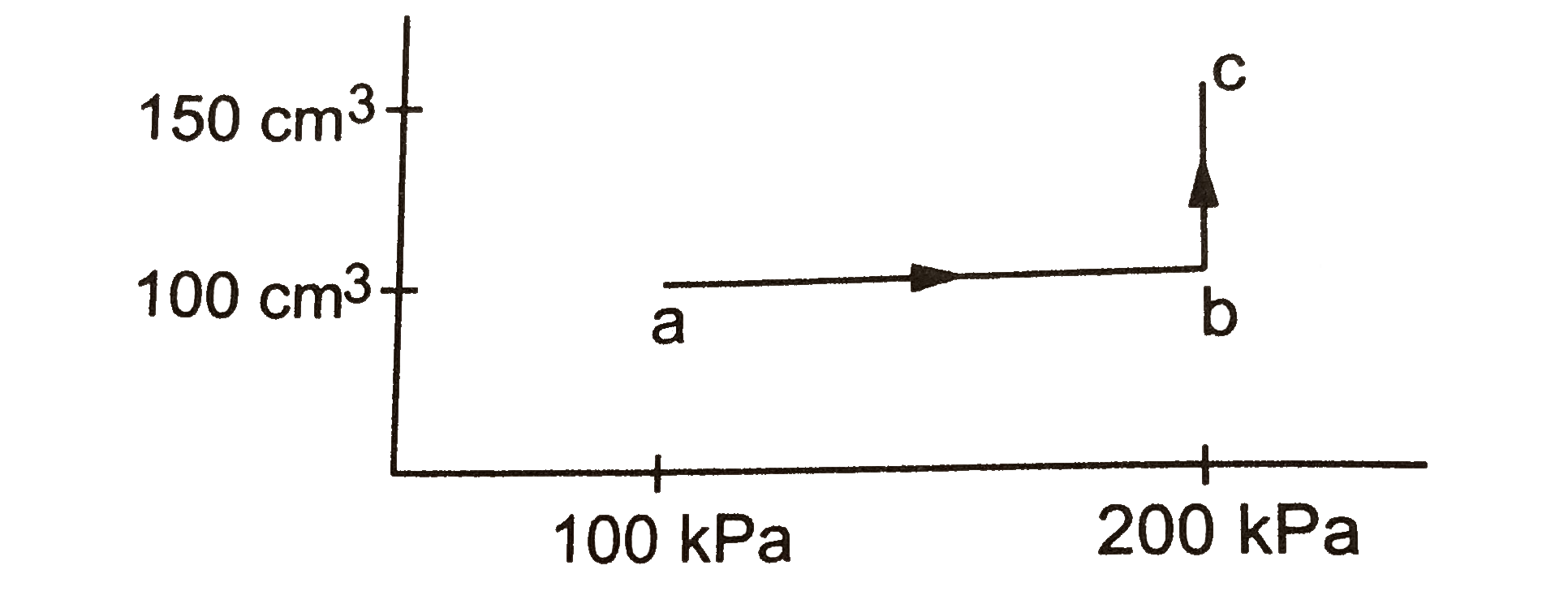
View SolutionAn ideal gas (γ=1.67) is taken through the process abc shown in figure . The temperature at the point a is 300k. Calculate (a) the temperature at b and c, (b) the work done in the process, ( c) the amount of heat supplied in the path ab and in the path bc and (d) the change in the internal energy of the gas in the process.
View Solution400 J of work is done on a gas to reduce its volume by compression adiabatically. What is the change in internal energy of the gas?
View SolutionIf work done by the system is 300 joule when 100 cal. Heat is supplied to it. The change in internal energy during the process is :-
View SolutionA diatomic gas is heated at constant pressure. If 105J of heat is given to the gas, find (a) the change in internal energy of the gas (b) the work done by the gas.
View Solution

