Which one of the following compounds is a superoxide?
KO2
BaO2
MnO2
NO2
The correct Answer is:B
To determine which compound is a superoxide, we need to understand the definition of a superoxide and analyze the oxidation states of oxygen in the given compounds.
1. Definition of Superoxide:
A superoxide is a compound that contains the superoxide ion (O2^−) where the oxidation state of each oxygen atom in the ion is -1/2.
2. Analyzing the Options:
We will evaluate each option to see if it contains the superoxide ion.
- Option 1: KO2 (Potassium Superoxide):
- Potassium (K) is in Group 1 and has a +1 oxidation state.
- The compound can be represented as K^+ and O2^2−.
- The two oxygen atoms in O2^2− have an average oxidation state of -1/2 (since 2 oxygen atoms share a -1 charge).
- Therefore, KO2 is a superoxide.
- Option 2: BaO2 (Barium Peroxide):
- Barium (Ba) is in Group 2 and has a +2 oxidation state.
- In BaO2, the two oxygen atoms share a -2 charge, leading to an average oxidation state of -1 for each oxygen.
- This indicates that BaO2 is a peroxide, not a superoxide.
- Option 3: MnO2 (Manganese Dioxide):
- Manganese (Mn) typically has a +4 oxidation state in MnO2.
- The two oxygen atoms each have a -2 oxidation state.
- Thus, MnO2 is a normal oxide, not a superoxide.
- Option 4: NO2 (Nitrogen Dioxide):
- Nitrogen (N) in NO2 has a +4 oxidation state.
- The two oxygen atoms each have a -2 oxidation state.
- Therefore, NO2 is also a normal oxide, not a superoxide.
3. Conclusion:
After analyzing all the options, we find that the only compound that qualifies as a superoxide is KO2.
Final Answer:
The compound that is a superoxide is KO2.
A superoxide is a compound that contains the superoxide ion (O2^−) where the oxidation state of each oxygen atom in the ion is -1/2.
We will evaluate each option to see if it contains the superoxide ion.
- Potassium (K) is in Group 1 and has a +1 oxidation state.
- The compound can be represented as K^+ and O2^2−.
- The two oxygen atoms in O2^2− have an average oxidation state of -1/2 (since 2 oxygen atoms share a -1 charge).
- Therefore, KO2 is a superoxide.
- Barium (Ba) is in Group 2 and has a +2 oxidation state.
- In BaO2, the two oxygen atoms share a -2 charge, leading to an average oxidation state of -1 for each oxygen.
- This indicates that BaO2 is a peroxide, not a superoxide.
- Manganese (Mn) typically has a +4 oxidation state in MnO2.
- The two oxygen atoms each have a -2 oxidation state.
- Thus, MnO2 is a normal oxide, not a superoxide.
- Nitrogen (N) in NO2 has a +4 oxidation state.
- The two oxygen atoms each have a -2 oxidation state.
- Therefore, NO2 is also a normal oxide, not a superoxide.
After analyzing all the options, we find that the only compound that qualifies as a superoxide is KO2.
The compound that is a superoxide is KO2.
Similar Questions
Knowledge Check
Which one of the following compounds is not aromatic?
A B
B C
C D
D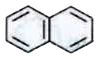
Which one of the following compounds gives carboxylic acid with HNO2 ?
A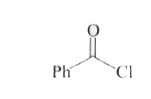 B
B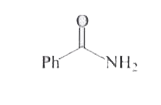 C
C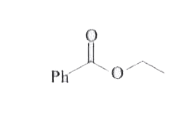 D
D
Which one of the following compounds is not a protoric acid?
View SolutionWhich one of the following compounds gives a positive iodoform test?
View SolutionWhich one of the following compounds gives carboxylic acid with HNO2?
View SolutionWhich one of the following compounds is not planar?
View SolutionWhich one of the following compounds is most acidic
View SolutionWhich one of the following compounds is most acidic
View Solution
