The total number of lone pair of electrons in N2O3 is
The correct Answer is:8
To determine the total number of lone pairs of electrons in N2O3, we will follow these steps:
Step 1: Determine the Lewis Structure of N2O3
- Start by identifying the number of valence electrons for each atom in the molecule.
- Nitrogen (N) has 5 valence electrons, and there are 2 nitrogen atoms: 2×5=10 electrons.
- Oxygen (O) has 6 valence electrons, and there are 3 oxygen atoms: 3×6=18 electrons.
- Total valence electrons = 10+18=28 electrons.
Step 2: Draw the Bonds
- Connect the two nitrogen atoms with a single bond.
- Connect one nitrogen atom to two oxygen atoms with double bonds.
- Connect the other nitrogen atom to one oxygen atom with a dative bond (where one atom provides both electrons).
Step 3: Assign Lone Pairs to Each Atom
- For the nitrogen atom with three bonds (the one connected to two oxygens with double bonds), it will have 1 lone pair.
- For the nitrogen atom connected to one oxygen with a dative bond, it has 0 lone pairs since it is involved in bonding.
- For the oxygen atoms:
- The oxygen atom involved in the dative bond will have 2 lone pairs.
- The two oxygen atoms involved in double bonds will each have 2 lone pairs.
Step 4: Count the Total Lone Pairs
- Nitrogen 1: 1 lone pair
- Nitrogen 2: 0 lone pairs
- Oxygen 1 (dative bond): 2 lone pairs
- Oxygen 2 (double bond): 2 lone pairs
- Oxygen 3 (double bond): 2 lone pairs
Now, summing these up:
- Total lone pairs = 1+0+2+2+2=7 lone pairs.
Conclusion
The total number of lone pairs of electrons in N2O3 is 7.
---
- Start by identifying the number of valence electrons for each atom in the molecule.
- Nitrogen (N) has 5 valence electrons, and there are 2 nitrogen atoms: 2×5=10 electrons.
- Oxygen (O) has 6 valence electrons, and there are 3 oxygen atoms: 3×6=18 electrons.
- Total valence electrons = 10+18=28 electrons.
- Connect the two nitrogen atoms with a single bond.
- Connect one nitrogen atom to two oxygen atoms with double bonds.
- Connect the other nitrogen atom to one oxygen atom with a dative bond (where one atom provides both electrons).
- For the nitrogen atom with three bonds (the one connected to two oxygens with double bonds), it will have 1 lone pair.
- For the nitrogen atom connected to one oxygen with a dative bond, it has 0 lone pairs since it is involved in bonding.
- For the oxygen atoms:
- The oxygen atom involved in the dative bond will have 2 lone pairs.
- The two oxygen atoms involved in double bonds will each have 2 lone pairs.
- Nitrogen 1: 1 lone pair
- Nitrogen 2: 0 lone pairs
- Oxygen 1 (dative bond): 2 lone pairs
- Oxygen 2 (double bond): 2 lone pairs
- Oxygen 3 (double bond): 2 lone pairs
- Total lone pairs = 1+0+2+2+2=7 lone pairs.
The total number of lone pairs of electrons in N2O3 is 7.
Similar Questions
Knowledge Check
Total number of lone pair of electrons in I−3 ion is
A3B6C9D12The total number of lone pairs of electrons in melamine is
A12B8C10D6
A total number of lone pairs of electrons in melamine is ….
View Solution Number of lone pairs are?
Number of lone pairs are?
View SolutionThe total number of lone pair of electrons in the given molecule is :
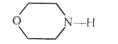
View SolutionThe total number of lone-pairs of electrons in melamine is.
View SolutionThe sum of total number of lone - pairs of electrons and sp3 hybridized nitrogen atoms in Melamine is
View SolutionThe total number of lone pair present in XeF4 is
View Solution
