What are 'X' and 'Y' respectively?
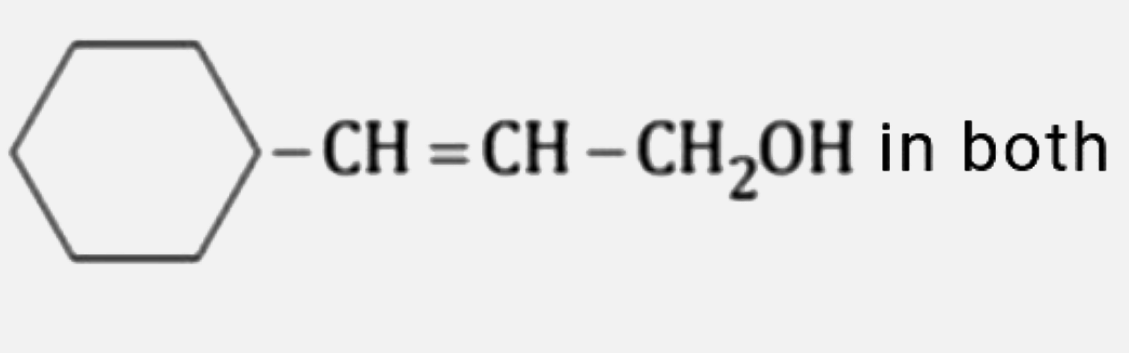 cases
cases
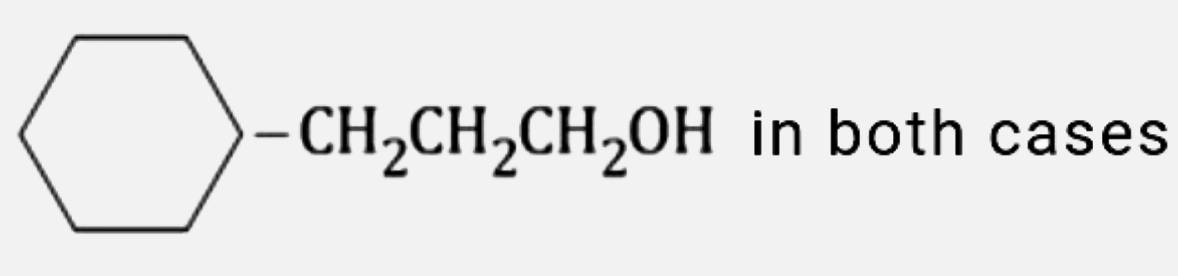
The correct Answer is:B
To solve the question, we need to identify the compounds 'X' and 'Y' formed from the reaction of the given reactant with two different reagents.
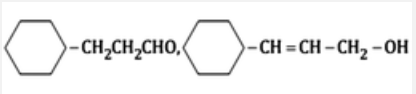
1. Identify the Reactant: The reactant given is CH=CHCHO, which is an aldehyde with a vinyl group (alkene) attached to it.
2. Reagent 1 - NaBH4: The first reagent is NaBH4 (sodium borohydride), which is known for selectively reducing aldehydes and ketones to their corresponding alcohols.
- When CH=CHCHO reacts with NaBH4, the aldehyde group (CHO) will be reduced to an alcohol (CH2OH).
- The alkene (C=C) remains unchanged.
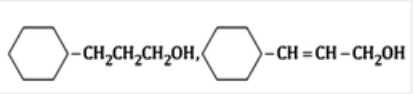
- Therefore, the product formed (let's denote it as 'X') is CH2=CH-CH2OH (an allylic alcohol).
3. Reagent 2 - H2/Pd: The second reagent is H2 in the presence of palladium (Pd), which can reduce both aldehydes and alkenes.
- When CH=CHCHO reacts with H2/Pd, both the aldehyde and the alkene will be reduced.
- The aldehyde (CHO) is reduced to CH2OH, and the alkene (C=C) is reduced to a saturated alkane (C-C).
- Therefore, the product formed (let's denote it as 'Y') is CH2-CH2-CH2OH (a saturated alcohol).
4. Conclusion:
- 'X' is the product from the reaction with NaBH4: CH2=CH-CH2OH.
- 'Y' is the product from the reaction with H2/Pd: CH2-CH2-CH2OH.
Final Answer:
- 'X' = CH2=CH-CH2OH (an allylic alcohol)
- 'Y' = CH2-CH2-CH2OH (a saturated alcohol)
- When CH=CHCHO reacts with NaBH4, the aldehyde group (CHO) will be reduced to an alcohol (CH2OH).
- The alkene (C=C) remains unchanged.
- Therefore, the product formed (let's denote it as 'X') is CH2=CH-CH2OH (an allylic alcohol).
- When CH=CHCHO reacts with H2/Pd, both the aldehyde and the alkene will be reduced.
- The aldehyde (CHO) is reduced to CH2OH, and the alkene (C=C) is reduced to a saturated alkane (C-C).
- Therefore, the product formed (let's denote it as 'Y') is CH2-CH2-CH2OH (a saturated alcohol).
- 'X' is the product from the reaction with NaBH4: CH2=CH-CH2OH.
- 'Y' is the product from the reaction with H2/Pd: CH2-CH2-CH2OH.
- 'X' = CH2=CH-CH2OH (an allylic alcohol)
- 'Y' = CH2-CH2-CH2OH (a saturated alcohol)
Similar Questions
In the reaction, xVO+yFe_2O_3rarr FeO+V_2O_5. What is the value of x and y respectively ?
View SolutionFigure showing different trophic level. If X,Y and Z is concentration of pesticide in level 5,3 and 1 respectively, what is the relationship of X and Z with Y. respectively
View SolutionX तथा Y समन्यूट्रॉनिक हैं। इनकी द्रव्यमान संख्या क्रमश: 70 तथा 72 है। यदि X की परमाणु संख्या 34 है?, तो Y की परमाणु है, ये निम्नलिखित में क्या कहलात हैं?
View SolutionGiven a function y=x2−2√x. What is rate of change in y with respect to x when x=I?
View SolutionGiven a function y=x2−2√x. What is rate of change in y with respect to x when x=I?
View SolutionGiven a function y=x2−2√x. What is rate of change in y with respect to x when x=I?
View Solution
What are the types of reactinos occurring in the formation of X, Y and Z respectively ?
View Solution
