Which of the following will exhibit geometrical isomerism?
Propene
Butene - 2
Butene - 1
1, 1 - dichloro butane
The correct Answer is:B
To determine which of the given compounds exhibits geometrical isomerism, we need to follow these steps:
Step 1: Understand the Conditions for Geometrical Isomerism
Geometrical isomerism occurs in compounds that have:
1. A carbon-carbon double bond (C=C).
2. Different atoms or groups attached to each carbon of the double bond.
Step 2: Analyze Each Option
Let's analyze each option to see if it meets the criteria for geometrical isomerism.
Option 1: Propene (C3H6)
- Structure: H2C=CH-CH3
- Analysis: The double bond is between the first two carbons. Both sides of the double bond have hydrogen atoms (H) attached, which are the same. Therefore, propene does not exhibit geometrical isomerism.
Option 2: But-2-ene (C4H8)
- Structure: H3C-CH=CH-CH3
- Analysis: The double bond is between the second and third carbons. The two carbons of the double bond have different groups attached:
- One carbon has a methyl group (CH3) and a hydrogen (H).
- The other carbon has a methyl group (CH3) and a hydrogen (H).
- This allows for both cis (where the similar groups are on the same side) and trans (where the similar groups are on opposite sides) isomers. Therefore, but-2-ene exhibits geometrical isomerism.
Option 3: But-1-ene (C4H8)
- Structure: H2C=CH-CH2-CH3
- Analysis: The double bond is between the first two carbons. The first carbon has two hydrogen atoms attached, and the second carbon has one hydrogen and one alkyl group (CH2-CH3). Since there are not two different groups on each side of the double bond, but-1-ene does not exhibit geometrical isomerism.
Option 4: 1,1-Dichlorobutane (C4H8Cl2)
- Structure: Cl-CH2-CH2-CH3
- Analysis: There is no double bond present in this compound. Since geometrical isomerism requires a double bond, 1,1-dichlorobutane does not exhibit geometrical isomerism.
Conclusion
Based on the analysis:
- Option 2 (But-2-ene) is the only compound that exhibits geometrical isomerism.
Final Answer
Option 2: But-2-ene exhibits geometrical isomerism.
---
Geometrical isomerism occurs in compounds that have:
1. A carbon-carbon double bond (C=C).
2. Different atoms or groups attached to each carbon of the double bond.
Let's analyze each option to see if it meets the criteria for geometrical isomerism.
- Structure: H2C=CH-CH3
- Analysis: The double bond is between the first two carbons. Both sides of the double bond have hydrogen atoms (H) attached, which are the same. Therefore, propene does not exhibit geometrical isomerism.
- Structure: H3C-CH=CH-CH3
- Analysis: The double bond is between the second and third carbons. The two carbons of the double bond have different groups attached:
- One carbon has a methyl group (CH3) and a hydrogen (H).
- The other carbon has a methyl group (CH3) and a hydrogen (H).
- This allows for both cis (where the similar groups are on the same side) and trans (where the similar groups are on opposite sides) isomers. Therefore, but-2-ene exhibits geometrical isomerism.
- Structure: H2C=CH-CH2-CH3
- Analysis: The double bond is between the first two carbons. The first carbon has two hydrogen atoms attached, and the second carbon has one hydrogen and one alkyl group (CH2-CH3). Since there are not two different groups on each side of the double bond, but-1-ene does not exhibit geometrical isomerism.
- Structure: Cl-CH2-CH2-CH3
- Analysis: There is no double bond present in this compound. Since geometrical isomerism requires a double bond, 1,1-dichlorobutane does not exhibit geometrical isomerism.
Based on the analysis:
- Option 2 (But-2-ene) is the only compound that exhibits geometrical isomerism.
Option 2: But-2-ene exhibits geometrical isomerism.
Similar Questions
Knowledge Check
Which of the following will not show geometrical isomerism ?
ACH3−C∣CH3=CH−CH2−CH3BCH3−CH∣CH3−CH=CH−CH2−CH3CCH3−CH=CH−CH3DCH3−CH2=CH−CH2−CH3Which of the following will not show geometrical isomerism ?
A B
B C
C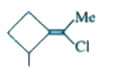 D
D
Which of the following will not show geometrical isomerism?
A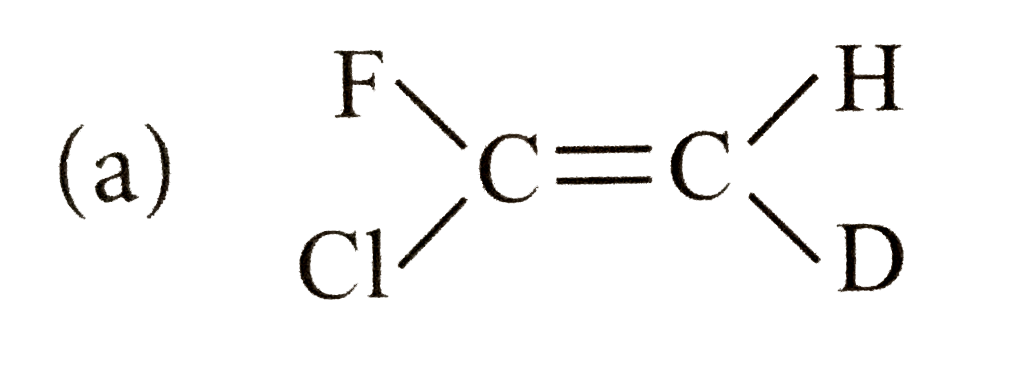 B
B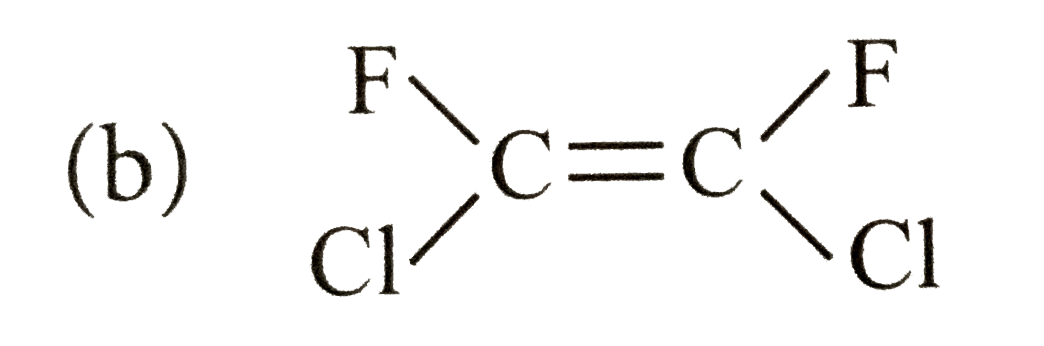 C
C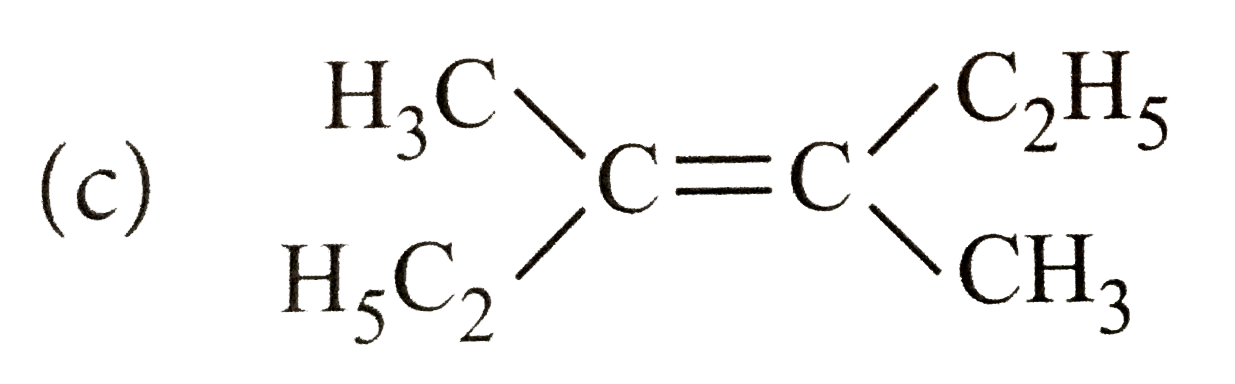 D
D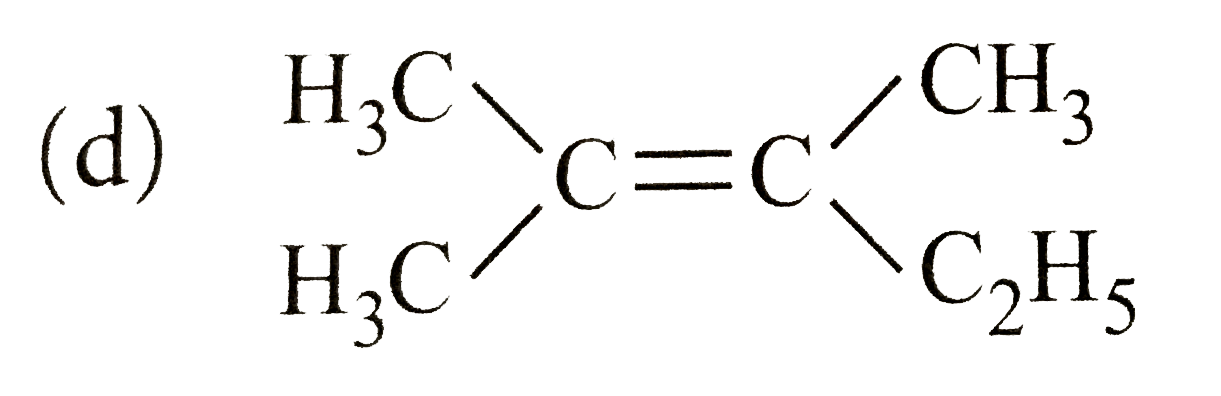
Which of the following will show geometrical isomerism ?
View SolutionWhich of the following will not show geometrical isomerism ?
View SolutionWhich of the following will not show geometrical isomerism?
View SolutionWhich of the following will not show geometrical isomerism?
View SolutionWhich of the following will not show geometrical isomerism?
View Solution

