Pb(CH3COO)2 gives …….colour with H2S
Orange
Red
Black
White
The correct Answer is:C
To solve the question regarding the color of the product formed when lead(II) acetate, Pb(CH3COO)2, reacts with hydrogen sulfide (H2S), we can follow these steps:
Step 1: Write the Reaction
The first step is to write the balanced chemical reaction for the interaction between lead(II) acetate and hydrogen sulfide.
Pb(CH3COO)2+H2S→PbS+2CH3COOH
Step 2: Identify the Products
From the reaction, we can see that lead(II) acetate reacts with hydrogen sulfide to produce lead(II) sulfide (PbS) and acetic acid (CH3COOH).
Step 3: Determine the Color of the Product
Next, we need to identify the color of lead(II) sulfide (PbS). It is well-known that lead(II) sulfide is a black solid.
Step 4: Conclusion
Thus, when lead(II) acetate reacts with hydrogen sulfide, the color observed is black due to the formation of lead(II) sulfide.
Final Answer
The answer to the question is that Pb(CH3COO)2 gives a black color with H2S.
The first step is to write the balanced chemical reaction for the interaction between lead(II) acetate and hydrogen sulfide.
From the reaction, we can see that lead(II) acetate reacts with hydrogen sulfide to produce lead(II) sulfide (PbS) and acetic acid (CH3COOH).
Next, we need to identify the color of lead(II) sulfide (PbS). It is well-known that lead(II) sulfide is a black solid.
Thus, when lead(II) acetate reacts with hydrogen sulfide, the color observed is black due to the formation of lead(II) sulfide.
The answer to the question is that Pb(CH3COO)2 gives a black color with H2S.
Similar Questions
Pb(CH3COO)2+H2S→
Product is:
View SolutionI2+Na2CO3 "soln." Hot−−→X+Y
If 'X' gives coloured ppt. with Pb(CH3COO)2 solution, then 'Y' will respond to which of the following ?
View SolutionThe reaction of compounf P with CH3MgBr (excess) in (C2H5_(2)O followed by addition of H2O give Q the compound Q on treatment with H2SO4 at 0∘ C gives R. the reaaction of R with CH3COCl in the presence of anhydrous AlCl3 in CH2Cl2 followed by treatment with H2O "produces compound S. [Et in compound P is ethyl group]
The reaction, Q to R and R TO S, are
View SolutionThe reaction of compounf P with CH3MgBr (excess) in (C2H5_(2)O followed by addition of H2O give Q the compound Q on treatment with H2SO4at 0∘ C gives R. the reaction of R with CH3COCl in the presence of anhydrous AlCl3inCH2Cl2 followed by treatment with H2O produces compound S. [Et in compound P is ethyl group] The product S is
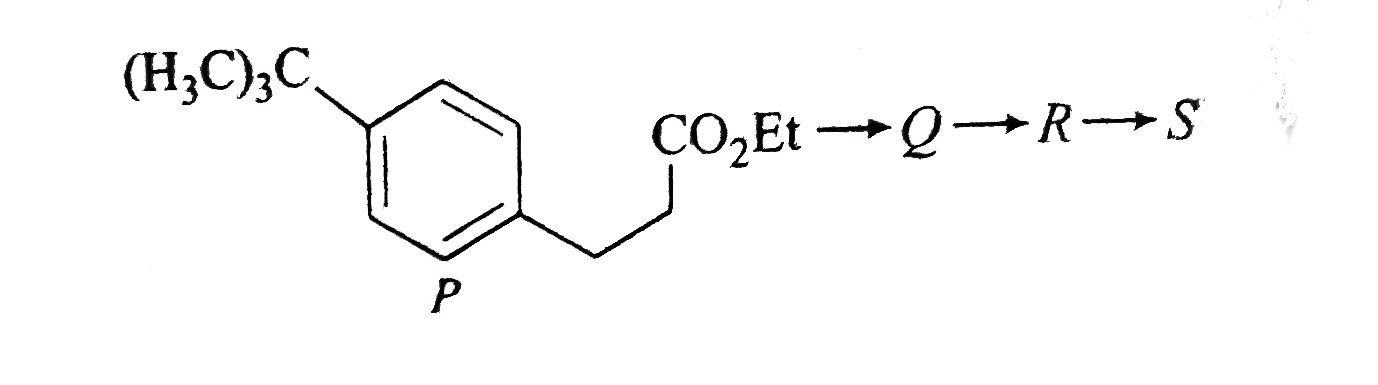
View SolutionConsider the fallowing statement :
(I) CH3OC⊕H2 is more stable that CH3CH⊕2
(II) Me3C⊕ is more stable than CH3CH2C⊕H2
(III) CH2=CH−C⊕H2 is more stable than CH3CH2C⊕H2
(IV) CH2=C⊕H is more stable thn CH3C⊕H2 of these statement:
View SolutionGive balanced equation for the conversions A and B .
Sulphide APb(CH3COO)2soln.−−−−−−−−−−−−→ Black precipitate
View SolutionCH3COO− ion is more stable than C2H5O− ion. Assign reason.
View SolutionWhich of the following compounds on oxidation with (CH3COO)4 Pd would give the product OHC(CH2)4CHO ?
View SolutionComplete the following reaction. Also give the colour change :
Pb(CH3COO)2Colourless+H2S→_______(__________)⏐⏐ ⏐↓+CH3COOH
View SolutionOn passing CrO2Cl2 in water and then adding (CH3COO)2Pb, the precipitate formed is
View Solution

