The reaction of p−HOC6H4COOH with excess Br2 forms
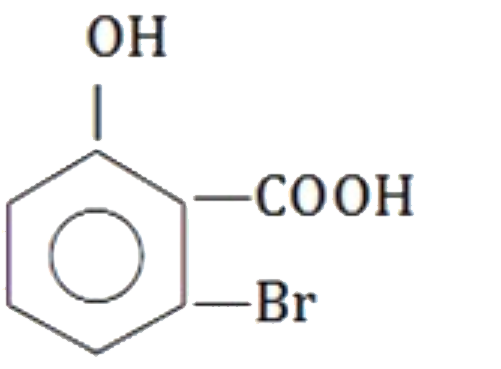
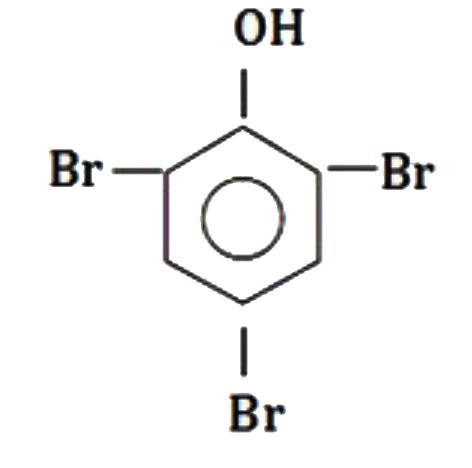
The correct Answer is:D
To solve the question regarding the reaction of para-hydroxybenzoic acid (p-HOC₆H₄COOH) with excess bromine (Br₂), we can follow these steps:
1. Identify the Structure of Para-Hydroxybenzoic Acid:
- Para-hydroxybenzoic acid has a hydroxyl group (-OH) and a carboxylic acid group (-COOH) attached to a benzene ring at the para position (1,4 positions).
- The chemical structure can be represented as:
OH∣C6H4−COOH
2. Reaction with Excess Bromine:
- When para-hydroxybenzoic acid reacts with excess bromine, the carboxylic acid group undergoes decarboxylation. This means that the -COOH group is removed, releasing carbon dioxide (CO₂).
- The reaction can be represented as:
p−HOC₆H₄COOH+Br₂→p−HOC₆H₄Br+CO₂+HBr
3. Formation of Brominated Product:
- After the decarboxylation, the resulting compound (which is para-bromophenol) can further react with excess bromine.
- The hydroxyl group on the benzene ring is ortho/para directing, which means that bromine will add to the 2 and 4 positions of the ring.
- This leads to the formation of 2,4,6-tribromophenol.
4. Final Product:
- The final product of the reaction is therefore 2,4,6-tribromophenol.
- The chemical structure can be represented as:
Br∣Br−C6H2(OH)−Br
5. Conclusion:
- Thus, the correct answer to the question is that the reaction of para-hydroxybenzoic acid with excess bromine forms 2,4,6-tribromophenol.
Final Answer:
The reaction of para-hydroxybenzoic acid with excess bromine forms 2,4,6-tribromophenol.
---
- Para-hydroxybenzoic acid has a hydroxyl group (-OH) and a carboxylic acid group (-COOH) attached to a benzene ring at the para position (1,4 positions).
- The chemical structure can be represented as:
OH∣C6H4−COOH
- When para-hydroxybenzoic acid reacts with excess bromine, the carboxylic acid group undergoes decarboxylation. This means that the -COOH group is removed, releasing carbon dioxide (CO₂).
- The reaction can be represented as:
p−HOC₆H₄COOH+Br₂→p−HOC₆H₄Br+CO₂+HBr
- After the decarboxylation, the resulting compound (which is para-bromophenol) can further react with excess bromine.
- The hydroxyl group on the benzene ring is ortho/para directing, which means that bromine will add to the 2 and 4 positions of the ring.
- This leads to the formation of 2,4,6-tribromophenol.
- The final product of the reaction is therefore 2,4,6-tribromophenol.
- The chemical structure can be represented as:
Br∣Br−C6H2(OH)−Br
- Thus, the correct answer to the question is that the reaction of para-hydroxybenzoic acid with excess bromine forms 2,4,6-tribromophenol.
The reaction of para-hydroxybenzoic acid with excess bromine forms 2,4,6-tribromophenol.
Similar Questions
Knowledge Check
The reaction RCH2CH2COOHRedP+Br2−−−−−−→R−CH2−CH(Br)−COOH
AReimer- Tiemann reactionBHell-Volhard - Zelinsky reactionCCannizzaro reactionDSandmeyer reaction .Reactions of C6H5CH2Br with aqueous sodium hydroxide follows……..
ASN1 mechanismBSN2 mechanismCAny of the above two depending upon the temperature of reactionDSaytzeff rule
Complete the following reaction:
CH3CH2COOHP,Br2−−−→[A](i)alc.KOH(excess)−−−−−−−−−−−−−→(ii)H+[B]
View SolutionComplete the following reaction:
CH3CH2COOHP,Br2−−−→[A](i)alc.KOH(excess)−−−−−−−−−−−−−→(ii)H+[B]
View SolutionComplete the following reaction:
CH3CH2COOHP,Br2−−−→[A](i)alc.KOH(excess)−−−−−−−−−−−−−→(ii)H+[B]
View SolutionThe reaction of Br2/PorCl2/P with carboxylic acid to form α- halogenated acid, is called
View SolutionThe phosphorous containing compound produced in the reaction of P4O6 with H2O is
View SolutionThe reaction
CH3COOH+Cl2redP−−→CICH2COOH+HCl is called
View Solution
