Substance A2B(g) can undergoes decomposition to form two set of products :
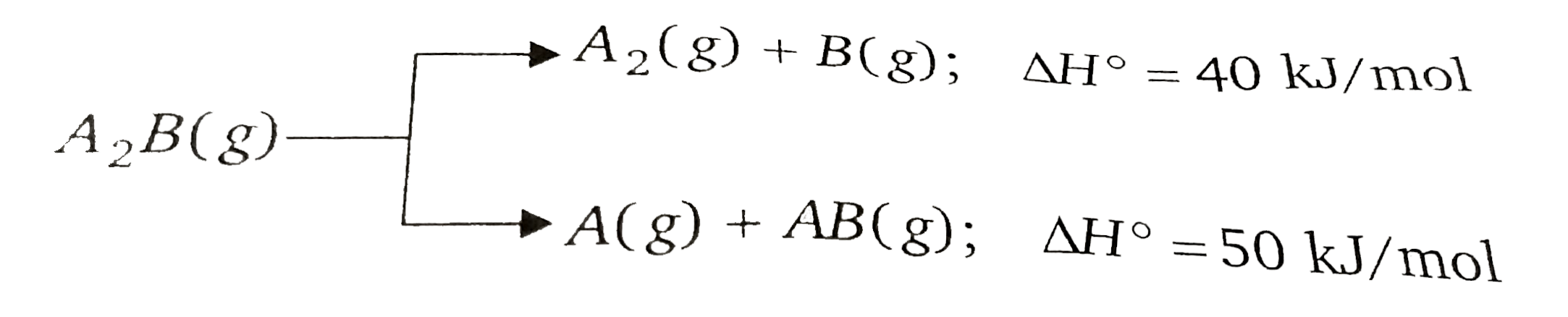
If the molar ratio of A2(g) to A(g) is 5:3 in a set of product gases, then the energy involved in the decomposition of 1 mole of A2B(g) is :
48.75kJ/mol
43.75kJ/mol
46.25kJ/mol
64.2kJ/mol
The correct Answer is:B
Step by step video solution for Substance A_(2)B(g) can undergoes decomposition to form two set of products : If the molar ratio of A_(2)(g) to A(g) is 5 : 3 in a set of product gases, then the energy involved in the decomposition of 1 mole of A_(2)B(g) is : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023সম্পর্কিত প্রশ্ন
Ethanol can undergo decompostion to form two sets of products.
If the molar ratio of C4H4toCH3CHO is 8:1 in a set of product gases, then the energy involved in the decomposition of 1 mole of ethanol is:
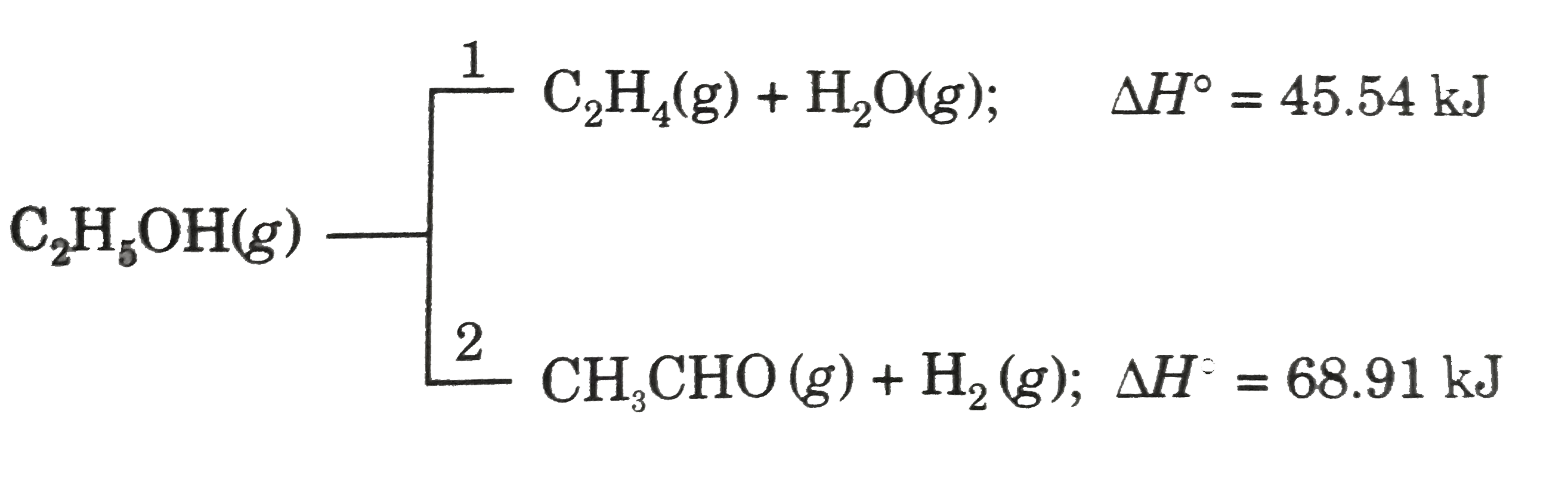
View SolutionThe yield of product in the reaction,
A2(g)+2B(g)⇔C(g)+QKJ
would be higher at:
View Solution
জ্ঞান পরীক্ষা করুন
If the first term of a G.P. a1,a2,a3 is unity such that 4a2+5a3 is least, then the common ratio of the G.P. is
A25B−25C−35D−15
A subtance undergoes first order decomposition involving two parallel first order reaction as :
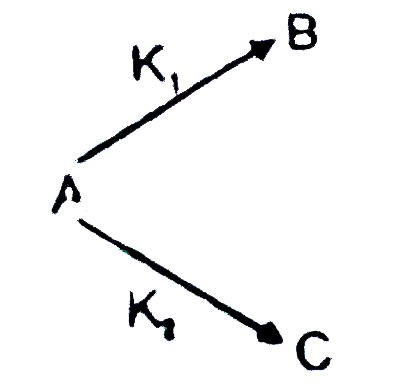
The mole percent of B in the products is :
View SolutionFor the Chemical reaction A2(g)+B2(g)⇔2AB(g) the amount of AB at equilibrium is affected by
View SolutionTwo substances, A and B were made to react to form a third substance, A2B according to the following reaction 2A+B→A2B.Which of the following statements concerning this reaction are incorrect?
(i) The product A2B shows the properties of substances A and B.
(ii) The product will always have a fixed composition.
(iii) The product so formed cannot be classified as a compound.
(iv) The product so formed is an element.
View SolutionA gaseous reaction A2(g)→B(g)+12C(g) shows increase in pressure form 100mm to 120mm in 5min. What is the rate of disappearance of A2 ?
View SolutionA gaseous reaction A2(g)→B(g)+12C(g) shows increase in pressure form 100mm to 120mm in 5min. What is the rate of disappearance of A2 ?
View SolutionLet A1, A2 and A3 be subsets of a set X. Which one of the following is correct ?
View SolutionFor the reaction A_((s)) + 2B_((g)) hArr 3C_((g)) . At constant pressure on addition of inert gas, the equilibrium state
View Solution
