Complete the subshell electronic configuration of He?Solution in Malayalam
Step by step video solution for Complete the subshell electronic configuration of He? by Chemistry experts to help you in doubts & scoring excellent marks in Class 10 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Identify the relation and complete: COOH-Acid, OH-…………….
View SolutionFill in the blanks: Haematite-Iron. Bauxite-…………….
View SolutionThe last subshell of an element is 3p and there are 3 electrons in it. Write the complete electronic configuration of the element.
View SolutionThe outer most subshell electronic configuration of an element is 3d54s2.
Write the complete subshell electronic configuration of this element.
View SolutionWrite the names and electronic configurations of the elements in which electrons enter into 3d subshell.
View SolutionWhen the last electron of an atom was filled in the 3d subshell, the subshell electron configuration was 3d8. Answer the questions related to third atom.
i) Complete subshell electronic configuration
ii) Atomic number
iii) Block
iv)Period number
v) Group number
View SolutionThe outermost subshell electronic configuration of an element is 3s23p4. Answer the following questions.
a) Write the complete subshell electronic configuration.
b) Find the valency of this element?
c) Is it metal or non-metal? Give reason.
View SolutionSubshell electronic configuration of sodium?
View SolutionWrite the subshell electronic configuration of Manganese(Mn).
View Solution[Ar] 3d5 4s2 is the sub shell electronic configuration of an element. Write down the complete subshell electronic configuration?
View SolutionIdentify the incorrect subshell electronic configuration.
View SolutionFind out atomic number,group, block period using subshell electronic configuration and then complete the table.
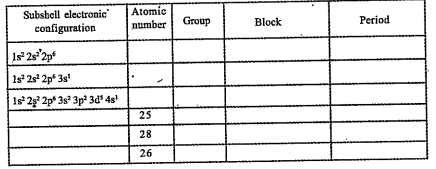
View SolutionThe subshell electronic configuration of two elementsends asfollows.(Symbols are not real) P−3s2 Q−3p4: Write the complete subshell electronic configuration.
View SolutionThe outer most subshell electronic configuration of an element is 3d^54s^2 : Write the complete subshell electronic configuration of this element.
View Solutionആറ്റത്തിന്റെ മൂന്നാമത്തെ ഷെല്ലിൽ 3 ഇലക്ട്രോണുകൾ ഉള്ള ഒരു മൂലകത്തിന്റെ സബ്ഷെൽ ഇലക്ട്രോൺ വിന്യാസം എഴുതുക.
View Solution