The figure of an incomplete periodic table is given below.
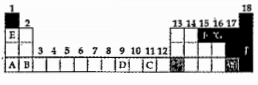
Which of these elements have 3 electrons
in their outermost p subshell ?Solution in Malayalam
Step by step video solution for The figure of an incomplete periodic table is given below. Which of these elements have 3 electrons in their outermost p subshell ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 10 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Identify the relation and complete: COOH-Acid, OH-…………….
View SolutionFill in the blanks: Haematite-Iron. Bauxite-…………….
View SolutionThe valence shell of the element X contains 1 electron in 5s subshell and below that shell, 4 electrons in 4d subshell. The element belongs to which group (IUPAC) of periodic table?
View SolutionElements having three incomplete outermost subshells called ……….
View SolutionSome elements belonging to second period of periodic table , and their atomic radii are given below .Observe them and write answers . which is the outermost orbit of alll these elements ?
View SolutionIf in long form of Periodic table : (i) Number of electrons in outermost "p" -subshell of most electronegative element is "x" . (ii) Group number of element having maximum electron affinity is 'y'. (iii) Number of electrons in outermost p-subshell of element having highest ionization energy is "z" (iv) Number of electrons in outermost s-subshell of strongest reducing element agent is "w" then determine value of "(y-x-z-3w)"
View SolutionIf in long form of Periodic table : (i) Number of electrons in outermost p-subshell of most electronegative element is 'X'.(ii) Group number of element having maximum electron affinity is 'Y'. (iii) Number of electrons in outermost p-subshell of element having highest ionization energy is 'z'.(iv) Number of electrons in outermost s-subshell of strongest reducing element agent is 'w'.then determine value of "(y-x-z-3w)"
View SolutionUse the periodic table to identify elements. (a) With 5 electrons in the outer subshell.
View SolutionThe electronic configuration of some element are given below Is the number of electrons in the outermost shell of these elements the same as that of the elements in the Table .
View SolutionAn incomplete form of the periodic table Is given below.(symbols are not real)
Which are transition elements?
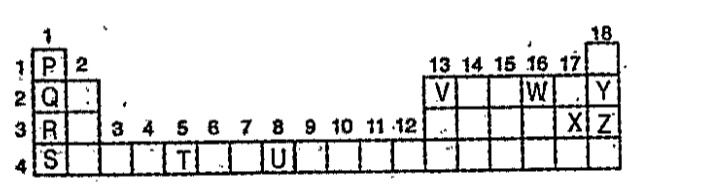
View SolutionAn incomplete form of the periodic table Is given below.(symbols are not real) Which element has the highest Ionisation energy?
View SolutionBelow are some elements of modern periodic table
4Be,9F,14Si,19K,20Ca
Select the element which has one electron in its outermost shell
View SolutionUse the periodic table to identify elements.
(a) With 5 electrons in the outer subshell.
View SolutionThe figure of an incomplete periodic table is given below. : Which one of these elements show -2 oxidation state?
View SolutionThe figure of an incomplete periodic table is given below. Which element has the highest atomic radius ? Which one has the least ?
View Solution