Three polymers are given below silk, rubber, PVC. Categorise each under separate heading based on intermolecular forces. Solution in Malayalam
Step by step video solution for Three polymers are given below silk, rubber, PVC. Categorise each under separate heading based on intermolecular forces. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Write the IUPAC names of the following compounds:
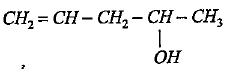
View SolutionWhich of the following concentration term is independent of temperature?
View SolutionAmong cellulose, poly viny chloride), nylon and nutural rubber, the polymer is which the intermolecular force of attraction is weakest is:
View SolutionAmong cellulose, poly (vinyl chloride), nylon and natural rubber, the polymer in which the intermolecular force of attraction is weakest is
View SolutionPolymer with strongest intermolecular force is :
View SolutionPolymer with weakest intermolecular force is :
View Solution(a) Write the names of the monomers of polymer used for making unbreakable crockery.
(b) Write the reaction involved in the preparation of neoprene
(c) Arrange the following polymers in decreasing order of intermolecular forces :
PVC, Nylon 66, Natural rubber.
View Solutionअन्तरअणुक बलों के आधार पर बहुलकों का वर्गीकरण कीजिए प्रत्येक प्रकार के बहुलक के विशिष्ट लक्षणों का वर्णन कीजिए ।
View SolutionDecreasing order of intermolecular forces of polymer is (i) Neoprene (ii) silk (iii) Buna-N (iv) melamine formaldehyde resin
View SolutionOrder of intermolecular forces of attraction in the polymers: (1) nylon 6 (2) neoprene and (3) PVC is-
View Solutionनिम्नलिखित बहुलकों को अन्तराआण्विक बलों के बढ़ते क्रम में व्यवस्थित कीजिए। इन्हें योगज तथा संघनन बहुलकों में भी वर्गीकृत कीजिए
नायलॉन-6, निओप्रीन, Pvc
View Solutionसेलुलोस, पॉलि (वाइनिल क्लोराइड), नायलॉन तथा प्राकृतिक रबड़ में से वह बहुलक जिसमें अन्तरआण्विक (intermolecular) आकर्षण बल दुर्बलतम् हैं, है
View SolutionWhat is the predominant intermolecular force of attraction between the adjacent chains of polymer molecules in natural rubber?
View SolutionArrange the following polymers in increasing order of intermolecular forces. Also calssify them as addition and condensation polymers :
Nylon - 6, neoprene, PVC.
View Solution(A) Natural rubber is an elastomer. (R) The intermolecular forces of attraction between the polymer chains are weak van der Waals forces.
View SolutionAmong cellulose, poly (vinyl chloride), nylon and natural rubber, the polymer in which the intermolecular force of attraction is weakest is
View Solution
