Choose the true statement:
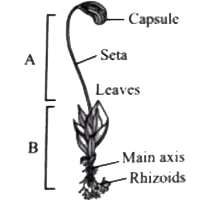
A
A is sporophyte and is independent
B
A is sporophyte and is dependent on B, which is gametophyte
C
B is sporophyte and is independent
D
B is sporophyte and is dependent on A for food, which is gametophyte
Video Solution
Text Solution
The correct Answer is:B
|
Answer
Step by step video & text solution for Choose the true statement: by Biology experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PLANT KINGDOMBOOK - PHYSICS WALLAHCHAPTER - PLANT KINGDOMEXERCISE - NEET PAST 10 YEARS QUESTIONS
36 Videos
- PLANT KINGDOMBOOK - PHYSICS WALLAHCHAPTER - PLANT KINGDOMEXERCISE - NEET PAST 10 YEARS QUESTIONS
36 Videos
- PLANT GROWTH AND DEVELOPMENTBOOK - PHYSICS WALLAHCHAPTER - PLANT GROWTH AND DEVELOPMENTEXERCISE - NEET PAST 10 YEARS QUESTIONS
17 Videos
- PRINCIPLES OF INHERITANCE AND VARIATION BOOK - PHYSICS WALLAHCHAPTER - PRINCIPLES OF INHERITANCE AND VARIATION EXERCISE - NEET Past 10 Years Questions
62 Videos
Similar Questions
Knowledge Check
Two identical capacitors are joined in parallel, charged to a potential V and then separated and then connected in series i.e. the positive plate of one is connected to negative of the other
AThe charges on the free plated connected together are destroyedBThe energy stored in the system increasesCThe potential difference between the free plates is 2V.DThe potential difference remains constant.The true statement is
Ahe order of colours in the primary and the secondary rainbows is the sameBThe intensity of colours in the primary and the secondary rainbows is the sameCThe intensity of light in the primary rainbow is greater and the order of colours is the same than the secondary rainbowDThe intensity of light for different colours in primary rainbow is greater and the order of colours is reverse than the secondary rainbowTrue statement is
Apoistive catalyst increases the rate of reaction.BDuring the course of the reaction, specific reaction rate remains constant.CRate constant always increases with rise in temperature whether the reaction is endothermic or exothermic.DAll are correct.

