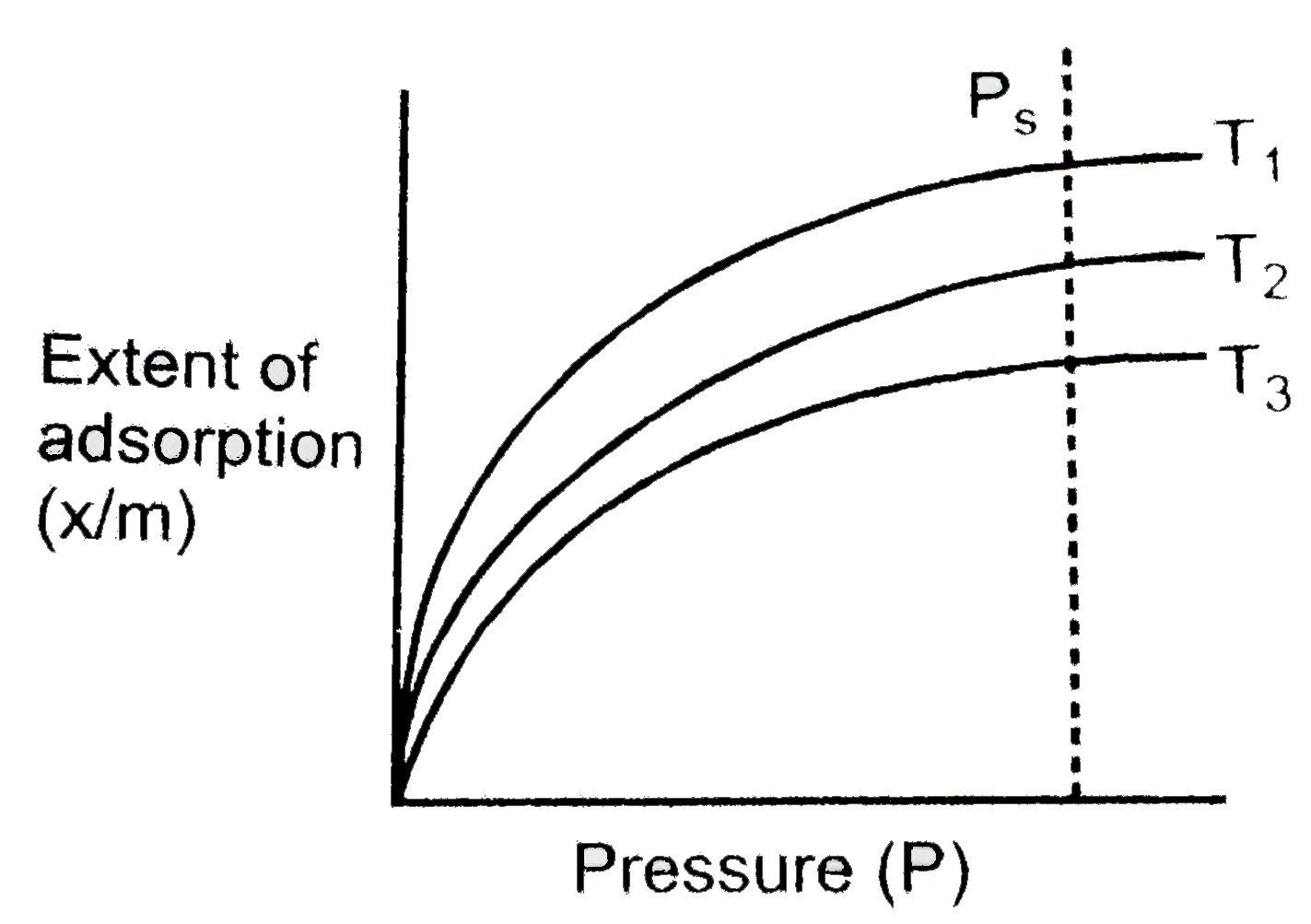
for the graph below, select correct order of temperture ?
 Solution in Punjabi
Solution in Punjabi
T1>T2>T3
T2>T3>T1
T3>T2>T1
T1>T2>T3
The correct Answer is:c
Step by step video solution for for the graph below, select correct order of temperture ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
5A→Product
In above reaction, half-life period is directly proportional to initial concentration of reactant. The initial rate of reaction is 400 moll−1−1min Then the order of reaction is
View SolutionStudy the charactristics of a population represented in the graphs below. Mark the correct graph that represents the type of selection that this population is likely to undergo
View SolutionStudy the charactristics of a population represented in the graphs below. Mark the correct graph that represents the type of selection that this population is likely to undergo
View SolutionFor the graph below select correct order of temperture :-
View SolutionSelect the correct order of CFSE (Delta) for the ions given below
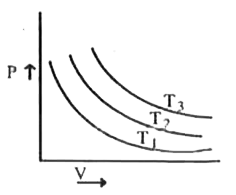
View SolutionA graph showing the variation of volume with temperture at constant pressure would look like
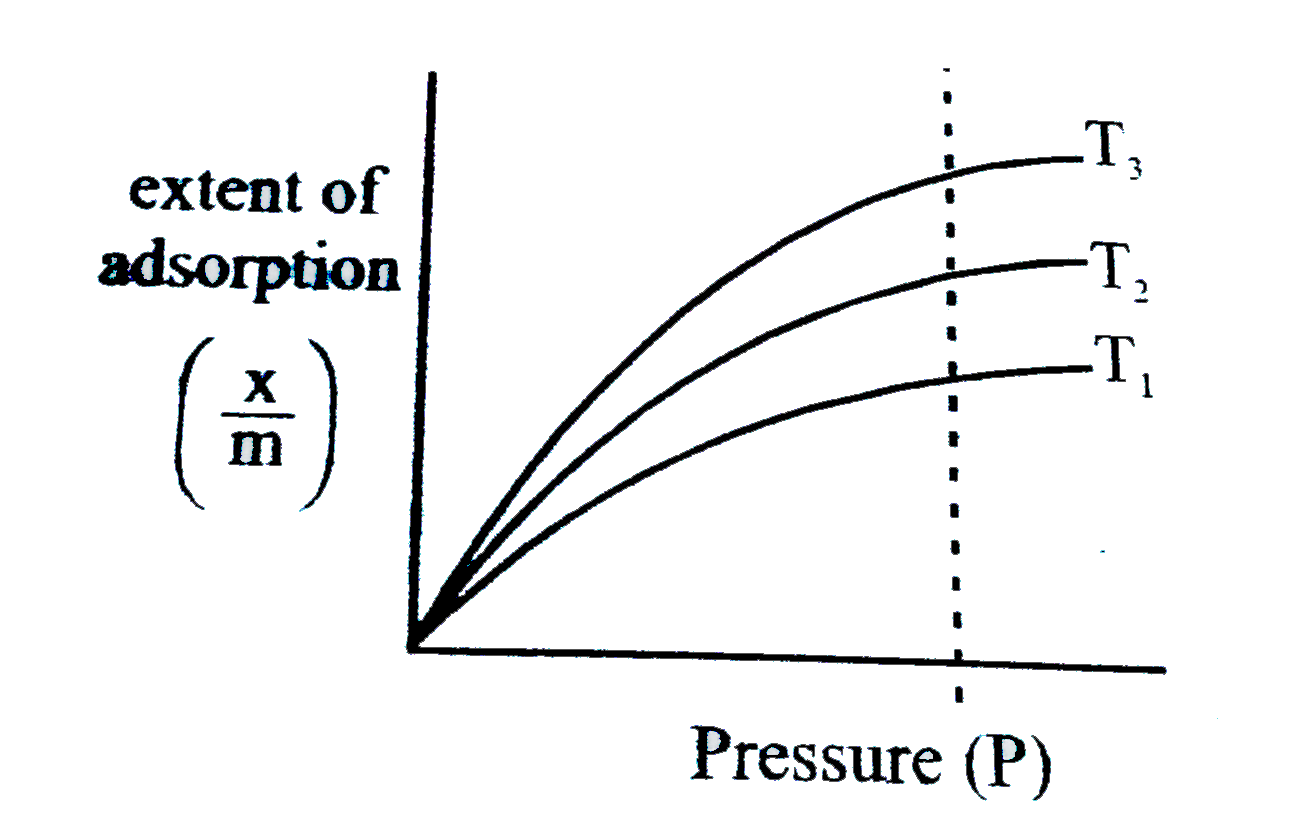
View SolutionFor the graph below, select correct order of temperature?
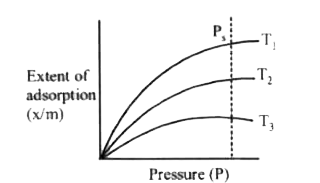
View SolutionFrom the graph the correct order of temperatures is:
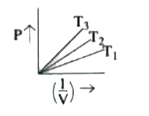
View SolutionFrom the graph the correct order of temperature is:
View SolutionFrom the graph the correct order of temperatures is
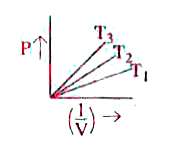
View SolutionFrom the graph the correct order of temperatures is
View SolutionFrom the graph the correct order of temperature is
View Solutionfor the graph below, select correct order of temperture ?
View SolutionStudy the charactristics of a population represented in the graphs below.
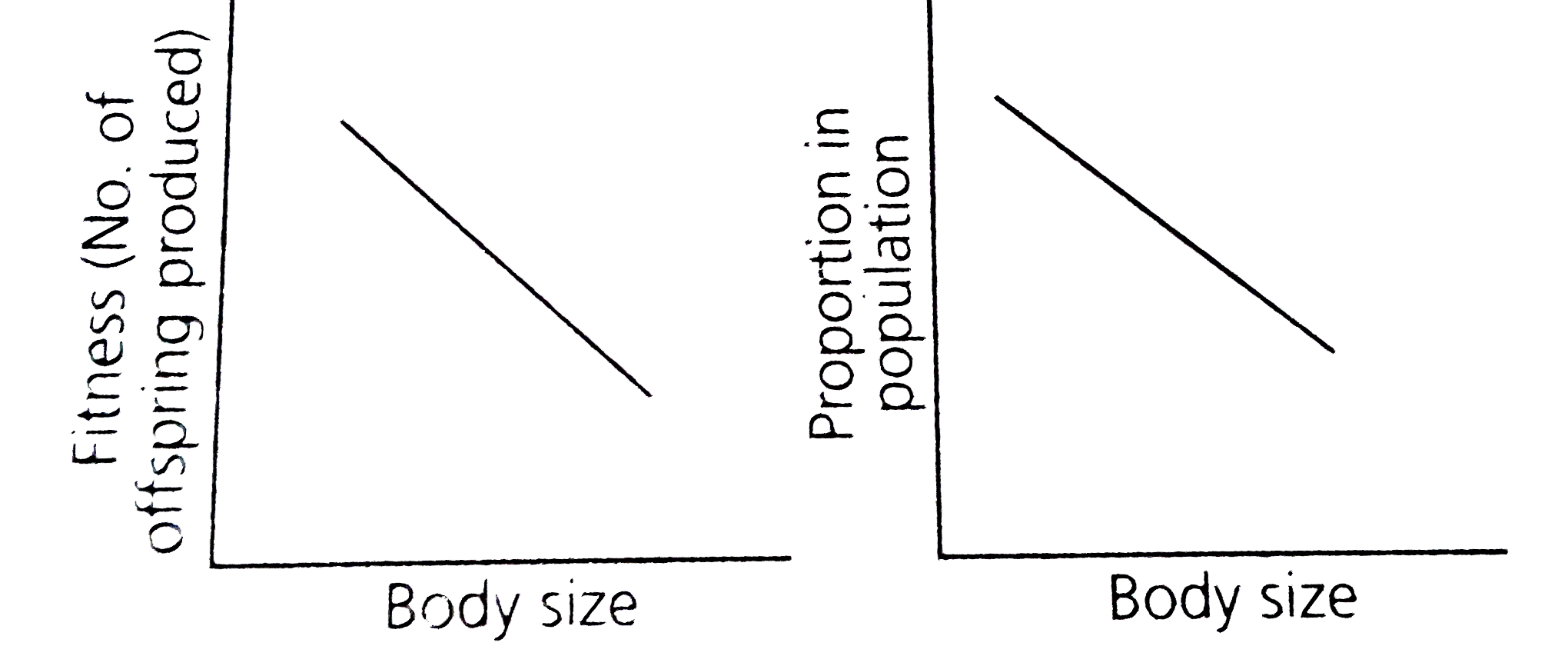
Mark the correct graph that represents the type of selection that this population is likely to undergo
View SolutionStudy the charactristics of a population represented in the graphs below. Mark the correct graph that represents the type of selection that this population is likely to undergo
View SolutionSelect the correct order for the strength of bases given below:
View Solution

