Which one is a wrong statement?
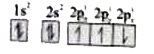
A
Total orbital angular momentum of electron in .s. orbital is equal to zero
B
An orbital is disignated by three quantum numbers while an electron in an atom is designated by four quantum numbers
C
The value of m for d2x is zero
D
The electronic configuration of N atom is
Text Solution
The correct Answer is:D
According to Hund.s Rule of maximum multiplicity, the correct electronic configuration of N-atom is
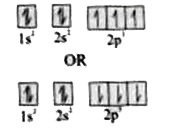
∴ Option (d) violates Hund.s Rule.
|
Topper's Solved these Questions
- STRUCTURE OF ATOM BOOK - PHYSICS WALLAHCHAPTER - STRUCTURE OF ATOM EXERCISE - Level-1
40 Videos
- STRUCTURE OF ATOM BOOK - PHYSICS WALLAHCHAPTER - STRUCTURE OF ATOM EXERCISE - Level-1
40 Videos
- STATES OF MATTERBOOK - PHYSICS WALLAHCHAPTER - STATES OF MATTEREXERCISE - NEET PAST 5 YEARS QUESTIONS
14 Videos
- SURFACE CHEMISTRYBOOK - PHYSICS WALLAHCHAPTER - SURFACE CHEMISTRYEXERCISE - NEET PAST 5 YEARS QUESTIONS
8 Videos
Similar Questions
Knowledge Check
Which one is wrong statement?
ATotal orbital angular momentum of electron in 's' orbital is equal to zeroBAn orbital is designated by three quantum numbers while an electron in an atom is designated by four quntum numbersCThe electronic configuration of N atoms is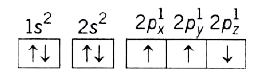 DThe value of m for d2Z is zero
DThe value of m for d2Z is zeroWhich one is wrong statement
AMucor has biflagellate zoosporesBHaploid endosperm is typical feature of gymnospermsCBrown algae have chlorophyll a and c and fucoxanthinDArchegonia are found in bryophyta, pteridophyta and gymnospermsWhich one is wrong statement ?
AMucor has biflagellate zoosporesBHaploid endosperm is typical feature of gymnospermsCBrown algae have chlorophyll a and c and fucoxanthinDArchegonia are found in Bryophyta, Pteridophyta and Gymnosperms.

