The shape of XeOF2 on the basis of VSEPR theory is:
A
See-saw
B
V-shaped
C
Trigonal planar
D
T-shaped
Text Solution
The correct Answer is:D
In XeOF2
Number of σ -bonds = 3
Number of lone pairs = 2
∴ Total number of pairs of electrons = 3 + 2 = 5
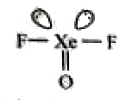
|
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:PHYSICS WALLAHChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:Level -2
Explore 48 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:PHYSICS WALLAHChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:NEET Past 5 Years Questions
Explore 38 Videos
- BIOMOLECULESBook:PHYSICS WALLAHChapter:BIOMOLECULESExercise:NEET PAST 5 YEARS QUESTIONS
Explore 13 Videos
- CHEMISTRY IN EVERDAY LIFE Book:PHYSICS WALLAHChapter:CHEMISTRY IN EVERDAY LIFE Exercise:NEET PAST 5 YEARS QUESTIONS
Explore 6 Videos
Similar Questions
Knowledge Check
The shape of XeF4 molecule according to VSEPR theory is
ASquare planarBSquare pyramidCTetrahedralDPyramidalThe shape of ClO−3 ion according to VSEPR theory is:
ATriangle planarBPyramidalCTetrahedralDsquare planar.
Deduce the molecular shape of BrF3 on the basis VSEPR theory.
View SolutionInterpret non-linear shape of H2S and non-planar shape of PCl3 on the basis of VSEPR theory.
View SolutionInterpret non-linear shape of H2S and non-planar shape of PCl3 on the basis of VSEPR theory.
View SolutionThe shape of ClO−3 ion according to VSEPR theory is:
View SolutionPredict the shape of ClF3 according to VSEPR theory.
View SolutionWhat will be the geometry of XeOF4 according to VSEPR theory ?
View Solution

