Which is the right structure of XeF4 ?
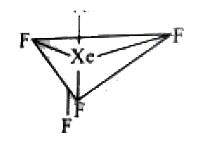
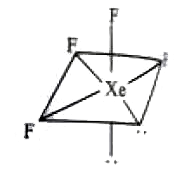
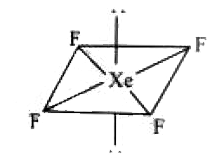
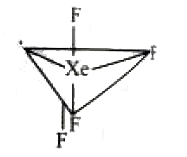
The correct Answer is:C
To determine the correct structure of XeF4, we can follow these steps:
Step 1: Determine the Valence Electrons
- Xenon (Xe) is a noble gas and has 8 valence electrons.
- Each Fluorine (F) atom contributes 1 valence electron, and since there are 4 fluorine atoms, they contribute a total of 4×1=4 valence electrons.
- Total valence electrons = 8+4=12 electrons.
Step 2: Calculate the Steric Number
- The steric number is calculated using the formula:
Steric Number=12(Valence Electrons of Central Atom+Number of Monovalent Atoms−Cationic Charge+Anionic Charge)
- Here, the central atom is xenon (Xe), which has 8 valence electrons, and there are 4 fluorine atoms (monovalent).
- Plugging in the values:
Steric Number=12(8+4+0+0)=12×12=6
Step 3: Determine the Geometry
- According to VSEPR theory, a steric number of 6 indicates an octahedral geometry.
- However, we need to account for the presence of lone pairs.
Step 4: Identify Bonding and Lone Pairs
- In XeF4:
- There are 4 bonding pairs (from the 4 Xe-F bonds).
- The remaining 2 electrons (from the total of 12) are lone pairs on the xenon atom.
- Thus, we have:
- Bonding pairs = 4
- Lone pairs = 2
Step 5: Determine the Molecular Shape
- With 4 bonding pairs and 2 lone pairs, the molecular geometry is derived from the octahedral arrangement.
- The lone pairs will occupy positions opposite each other to minimize repulsion, resulting in a square planar shape for the bonding pairs.
Step 6: Final Structure
- The final structure of XeF4 is square planar with the fluorine atoms in the same plane and the lone pairs positioned above and below the plane.
Conclusion
The correct structure of XeF4 is square planar.
---
- Xenon (Xe) is a noble gas and has 8 valence electrons.
- Each Fluorine (F) atom contributes 1 valence electron, and since there are 4 fluorine atoms, they contribute a total of 4×1=4 valence electrons.
- Total valence electrons = 8+4=12 electrons.
- The steric number is calculated using the formula:
Steric Number=12(Valence Electrons of Central Atom+Number of Monovalent Atoms−Cationic Charge+Anionic Charge)
- Here, the central atom is xenon (Xe), which has 8 valence electrons, and there are 4 fluorine atoms (monovalent).
- Plugging in the values:
Steric Number=12(8+4+0+0)=12×12=6
- According to VSEPR theory, a steric number of 6 indicates an octahedral geometry.
- However, we need to account for the presence of lone pairs.
- In XeF4:
- There are 4 bonding pairs (from the 4 Xe-F bonds).
- The remaining 2 electrons (from the total of 12) are lone pairs on the xenon atom.
- Thus, we have:
- Bonding pairs = 4
- Lone pairs = 2
- With 4 bonding pairs and 2 lone pairs, the molecular geometry is derived from the octahedral arrangement.
- The lone pairs will occupy positions opposite each other to minimize repulsion, resulting in a square planar shape for the bonding pairs.
- The final structure of XeF4 is square planar with the fluorine atoms in the same plane and the lone pairs positioned above and below the plane.
The correct structure of XeF4 is square planar.
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:PHYSICS WALLAHChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:NEET Past 5 Years Questions
Explore 38 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:PHYSICS WALLAHChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:NEET Past 5 Years Questions
Explore 38 Videos
- BIOMOLECULESBook:PHYSICS WALLAHChapter:BIOMOLECULESExercise:NEET PAST 5 YEARS QUESTIONS
Explore 13 Videos
- CHEMISTRY IN EVERDAY LIFE Book:PHYSICS WALLAHChapter:CHEMISTRY IN EVERDAY LIFE Exercise:NEET PAST 5 YEARS QUESTIONS
Explore 6 Videos
Similar Questions
Which of the following set of molecules will have zero diplole moment?
View SolutionWhich of the following set of molecules will have zero diplole moment?
View SolutionWhich of the following is a basic amino acid?
View SolutionWhich of the following is a cationic detergent?
View SolutionDraw the structure of XeF4 molecule.
View SolutionWhich structure for XeO_3 and XeF_4 are consistent with the VSEPR model
View SolutionDraw the structure of XeF4 molecule.
View SolutionExplain the structure of XeF_4.
View SolutionDraw the structure of XeF4 molecule.
View SolutionDraw the structure of XeF4.
View SolutionDraw the structure of XeF4 molecule.
View SolutionDraw the structure of XeF2, XeF4 and XeF6.
View SolutionGive hybridization and draw structure of XeF4.
View SolutionWhich structures for XeO_3 , and XeF_4 are consistent with the VSEPR model ?
View SolutionDraw the structure of the XeF_4 .
View Solution
