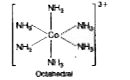
Discuss the struchure of [Co(NH3)6]3+ ion on the basis of valence bond theory. Whether it is an inner orbital or outer orbital complex ion ?Solution in Odia
Structure of Co(NH3)6]3+. Here, Co is our central metal element. Configuration of Co (atom) is [Ar]3d74s2.
Configuration of Co3+ ion = [Ar]3d6.i.e

∴Co3+ ion has 4 unpaird electrons in 3d-subshell and NH3 is a strong ligand.
∴ 3d-electrons go for pairing and configuration becomes:
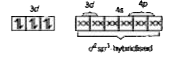
Thus, Co3+ in [Co(NH3)6]3+ is in d2sp3 (inner orbital octahendral complex) and is diamagnetic in nature due t0 absence of unpaired electrons
टॉपर्स ने हल किए ये सवाल
- QUESTION PAPER 2019Book:ARIHANT PUBLICATIONChapter:QUESTION PAPER 2019Exercise:GROUP C
Explore 11 Videos
- QUESTION PAPER 2019Book:ARIHANT PUBLICATIONChapter:QUESTION PAPER 2019Exercise:GROUP C
Explore 11 Videos
- POLYMERSBook:ARIHANT PUBLICATIONChapter:POLYMERSExercise:CHAPTER PRACTICE ( LONG ANSWER TYPE QUESTIONS )
Explore 3 Videos
- SAMPLE PAPER 1 Book:ARIHANT PUBLICATIONChapter:SAMPLE PAPER 1 Exercise:GROUP C
Explore 14 Videos
समान प्रश्न
What are oil in water and water in oil type of emulsions ? Give one example of cach type.
View SolutionWhat are oil in water and water in oil type of emulsions ? Give one example of cach type.
View SolutionWhat are natural and synthetic polymers? Give two examples of each type.
View SolutionA strip of nickel metal is dipped in a 1 molar solution of Ni(NO3)2 and a strip of silver metal is dipped in a 1 molar solution of AgNO3. An electrochemical cell is created when the two solutions are joined by salt bridge and two strips are joined by wire to a voltmeter.
Answer the following questions.
Write the balanced equation for the overall cell reaction and calculate the cell potential.
View SolutionExplain [Co(NH_3)_6]^(3+) is an inner orbital complex whereas [Ni(NH_3)_6]^(2+) is an outer orbital complex.
View Solution[Co(NH3)6]3+একটিঅভ্যন্তরীণকক্ষকঘটিত(∈≠rorbital)কমপ্লেক্সকিন্তু[Ni(NH_3)_6]^(2+) একটি বহিস্থ কক্ষকঘটিত (outer orbital) কমপ্লেক্স। ব্যাখ্যা করো।
View SolutionExplain [Co(NH_3)_6 ]^(3+) is an inner orbital complex whereas [Ni(NH_3)_6]^(2+) is an outer orbital complex.
View SolutionExplain [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
View SolutionWhy [Co(NH3)6]3+ is an inner orbital complex where is [Ni(NH3)6]2+ is an outer orbital complex ?
View SolutionExplain [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
View SolutionExplain [Co(NH_3)_6]^(3+) is an inner orbital complex whereas [Ni(NH_3)_6]^(2+) is an outer orbital complex.
View SolutionDiscuss the struchure of [Co(NH3)6]3+ ion on the basis of valence bond theory. Whether it is an inner orbital or outer orbital complex ion ?
View Solution[Co(NH_3)_6]^3+ is an inner orbital complex whereas [Ni(NH_3)_6]^(2+) is an outer orbital complex.Explain.
View SolutionOne the basis of valence bond theory explain the structure of [Co(NH3)6]3+ ion
View SolutionUsing valence bond theory of complexes explain the geometry and diamagnetic nature of the ion, [Co(NH3)6]3+.
View Solution
