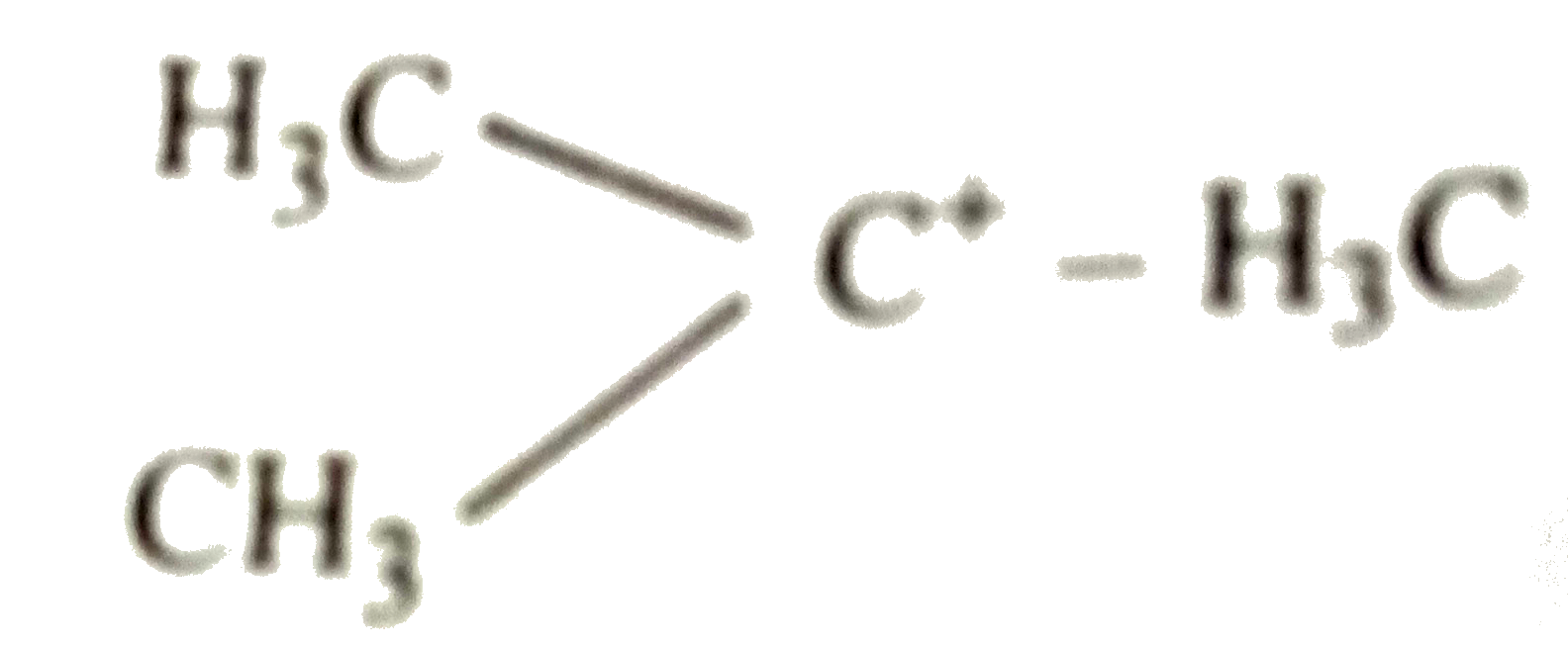
Among the following, the intensive property is (properties are):
A
Mass
B
Volume
C
Surface tension
D
Enthalpy
Video Solution
Text Solution
The correct Answer is:C
Extensive properties The properties of the system which depends upon the quantity of matter contained in it are called extensive properties e.g., mass, volume, energy. heat capacity etc.
Intensive properties: The properties which are independent of the quantity of matter present in it are called intensive properties e.g., temperature, pressure, refractive index, viscosity, specific heat, density, surface tension etc.
|
Topper's Solved these Questions
- THERMODYNAMICSBook:PHYSICS WALLAHChapter:THERMODYNAMICSExercise:NEET PAST 5 YEARS QUESTIONS
Explore 10 Videos
- THERMODYNAMICSBook:PHYSICS WALLAHChapter:THERMODYNAMICSExercise:NEET PAST 5 YEARS QUESTIONS
Explore 10 Videos
- THE SOLID STATEBook:PHYSICS WALLAHChapter:THE SOLID STATEExercise:NEET PAST 10 YEARS QUETSIONS
Explore 8 Videos
Similar Questions
Knowledge Check
A mong the following the intensive property is (properties are)
Amolar conductivityBelectromotive forceCresistanceDheat capacityAmong the following, the intersive property is (properties are) :
Amolar conductivityBelectromotive forceCresistanceDheat capacityAmong the following, the intensive property is (properties are):
Amolar conductivityBelectromotive forceCresistanceDheat capacity