A zero order reaction is one
A
in which the difference of concentration of product and reactant is zero
B
in which concentration of the reactants do not change with time.
C
in which one of the reactants is ine large excess
D
in which reactants do not react
Text Solution
The correct Answer is:B
Zero order reaction is a chemical reaction, where the rate does not vary with the increase or decrease in the concentration of the reactants.
|
Similar Questions
Knowledge Check
The rate constant for a zero order reaction is
Ak=C02tBk=C0−CttCk=ln.C0−CttDk=C0CtThe plot that represents the zero order reaction is:
A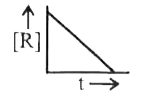 B
B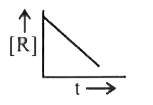 C
C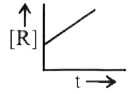 D
D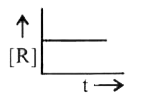
If half-lives of a first order and zero order reactions are same, then the ratio of the initial rates of the first order reaction to that of zero order reaction is
A1/0.693B2×0.693C2/0.693D6.93
For a zero order reaction will the molecularity be equal to zero ? Explain.
View SolutionHalf life of a first order reaction and a zero order reaction are same. Then the ratio of the initial rate of the first order reaction to that of zero order reaction is
View SolutionFor a zero order reaction
View SolutionFor a zero order reaction
View SolutionIn a zero order reaction:
View Solution

