Bond angle in PH+4 is higher than that in PH3 Why?
Text Solution
In both PH3 and PH+4 P is sp-hybridised. In PH3 ,there is a lone pair of electron whereas in PH+4 , all the 4 orbitals are bonded. Due to lone pair-bond pair repulsion in PH3, the bond angle is less than 109.5∘
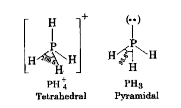
|
Similar Questions
Bond angle in PH+4 is higher than that in PH3. Why?
View SolutionBond angle in PH_(4)^(+) is higher than in PH_(3). Why?
View SolutionBond angle in PH_(4^(+) is higher than in PH_3 Why?
View SolutionBond angle in PH+4 is higher than that in PHz. Why?
View SolutionBond angle in PH_4^(+) is higher than that in PH_3 . Why?
View SolutionBond angle in PH+4 is higher than that in PH3.Why ?
View SolutionBond angle in is PH+4 higher than that in PH3
View SolutionBond angle in PH+4 is higher than that is PH3.
View Solution

