What is the degree of hardness of a water sample containing 5×10−3g/L of CaCO3?Solution in Malayalam
Hardness ( in pm of CaCO3)=5.0×10−3100×106=5p±
टॉपर्स ने हल किए ये सवाल
- SOME BASIC CONCEPTS OF CHEMISTRYBOOK - BRILLIANT PUBLICATIONCHAPTER - SOME BASIC CONCEPTS OF CHEMISTRYEXERCISE - LEVEL-I
55 Videos
- SOME BASIC CONCEPTS OF CHEMISTRYBOOK - BRILLIANT PUBLICATIONCHAPTER - SOME BASIC CONCEPTS OF CHEMISTRYEXERCISE - LEVEL-II (ASSERTION REASON TYPE QUESTIONS)
7 Videos
- SOME BASIC CONCAPTS OF CHEMISTRY BOOK - BRILLIANT PUBLICATIONCHAPTER - SOME BASIC CONCAPTS OF CHEMISTRY EXERCISE - LEVEL-III (Linked Comprehension Type)
6 Videos
- STATES OF MATTER BOOK - BRILLIANT PUBLICATIONCHAPTER - STATES OF MATTER EXERCISE - LEVEL III (Linked Comprehension Type )
9 Videos
समान प्रश्न
Which of the following is a characteristic of both mixtures and compounds?
View SolutionAssertion : Equal moles of different substances contain same number of constituent particles
Reason : Equal weights of different substances contain the same number of constituent particles
View SolutionIsotopes are the atoms of same element: they have same atomic number but different mass numbers. Isotope have different number of neutrons in their nucleus. If an element exists in two isotopes having atomic mas 'a' and 'b' in the ratio m:n, then average atomic mass will be m×a+n×bm+n
Different isotopes of same element have same position in the periodic table. The elements which have single isotope are called monoisotopic elements. Greater is the percentage composition of an isotope, more will be its abundance in nature.
Atomic mass of boron is 10.81. It has two isotopes namely115Bandx5B with their relative abundance of 80 and 20% respectively. The value of x is
View Solutionwhich of the following statements is correct?
View Solution10L of hard water requires 0.28g of line (CaO) for removing hardness. Calculate the temporary hardness in ppm of CaCO3.
View SolutionHardness of a water sample is 100ppm CaCO3. Thus molarity of CaCO3 is
View SolutionOne litre of a sample of hard water contain 4.44mgCaCl2and1.9mgofMgCl2. What is the total hardness in terms of ppm of CaCO3?
View SolutionOne kilogram sample of hard water contains 4.44 mg of CaCl2 and 1.9 mg of NaCl. The total hardness in tems of ppm of CaCO3 is :
View SolutionA sample ofsea water contains 5×10−3g of dissolved oxygen in 1 kg of the sample. The concentration of O2 in that sea water sample in ppm is
View Solution1L of a sample of hard water contains 1 mg of each of CaCl_(2) and MgCl_(2) . Express the harnedd of the sample in ppm, in terms of CaCO_(3) .
View SolutionA sample of hard water contains 1 xx 10^(-3) mole of CaCl_2 per 10kg of water. Calculate the degree of hardness of water sample
View Solution100Kg of a sample of water contained 6 g of MgSO4 . The degree of hardness
View SolutionOne litre of a sample of hard water contain 4.44mg CaCl_(2) and 1.9mg "of" MgCl_(2) . What is the total hardness in terms of ppm of CaCO_(3)?
View SolutionA sample of hard water contains 1×10−3 mole of CaCl2 per 10kg of water. Calculate the degree of hardness of water sample
View SolutionA hard water sample contains 162 ppm of calcium bicorbonate only. Find out the volume of standard soap solution (1ml=10−3gCaCO3) to be added to the 100mlf of hard water sample.
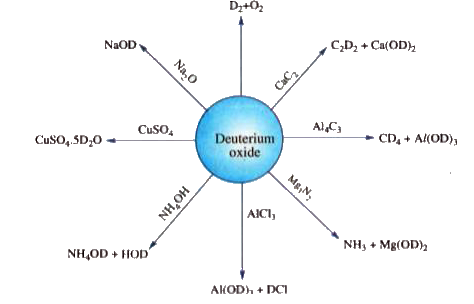
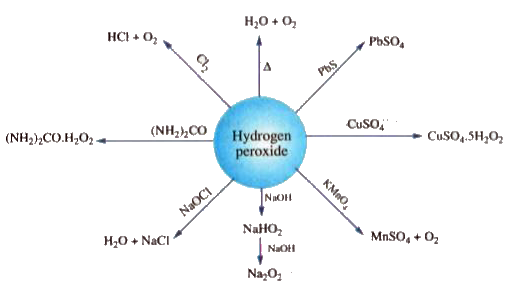
View Solution10L of hard water requires 0.28g of line (CaO) for removing hardness. Calculate the temporary hardness in ppm of CaCO3.
View Solution

