Consider the given plots for a reaction obeying Arrhenius equation (0°C < T < 300°C): (k and Ea are rate constant and activation energy, respectively)
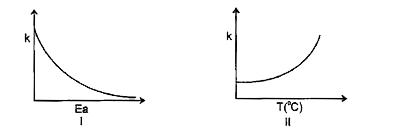
Choose the correct option :Solution in Malayalam
1) Both I and II are wrong
2) I is wrong but II is right
3) Both l and II are correct
4) I is right but II is wrong
Step by step video solution for Consider the given plots for a reaction obeying Arrhenius equation (0°C < T < 300°C): (k and E_a are rate constant and activation energy, respectively) Choose the correct option : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL KINETICS BOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL KINETICS EXERCISE - QUESTION (LEVEL - II) (Assertion - Reason)
4 Videos
- CHEMICAL KINETICS BOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL KINETICS EXERCISE - QUESTION
19 Videos
- CHEMICAL KINETICS BOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL KINETICS EXERCISE - QUESTION (LEVEL - l) (HOMEWORK) (ASSERTION- REASON)
6 Videos
- CARBOXYLIC ACIDSBOOK - BRILLIANT PUBLICATIONCHAPTER - CARBOXYLIC ACIDSEXERCISE - LEVEL-II (Assertion -Reason)
4 Videos
- CO-ORDINATION COMPOUNDS AND ORGANOMETALLICSBOOK - BRILLIANT PUBLICATIONCHAPTER - CO-ORDINATION COMPOUNDS AND ORGANOMETALLICSEXERCISE - Level-II (Assertion- Reason)
4 Videos
Similar Questions
Assertion: Precipitation of silver chloride occurs instantaneously by mixing of aqueous solutions of silver nitrate and sodium chloride
Reason: Ionic relations occur very fast
View SolutionA chemical reaction 2A→4B +C in gas phase occurs in a closed vessel the concentration of B is found to increase by 2.5×10−3molL−1 in 5 second calculate (i) the rate of appearance of B (ii) the rate of disappearance of A
View SolutionAssertion : Concentration of reactant in zero order reaction is constant.
Reason : For zero order reaction A→B , successive half life of reaction decreases with the progress of the reaction.
View SolutionAssertion : p-chlorobenzoic acid is more acidic than p-fluorobenzoic acid.
Reason : +R effect of fluorine is higher than that of chlorine due to the matching size of carbon and fluorine.
View SolutionAssertion : The complex ion [Ni(en)3]2+ has lesser stability than [Ni(NH3)6]2+
Reason : ln[Ni(en)3]2+, the geometry is square bipyramidal
View SolutionConsider the given plots for a reaction obeying Arrhenius equation (0^(@)CltTlt300^(@)C) : (k and E_(a) are rate constant and activation energy, respectively)
View SolutionConsider the given plots for a reaction obeying Arrhenius equation (0∘C<T<300∘C) : (kandEa are rate constant and activation energy, respectively) Choose the correct option :
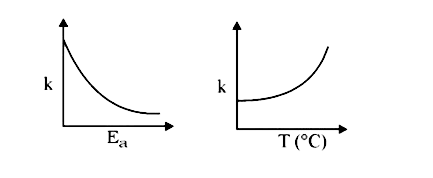
View SolutionRate constant k of a reaction varies with temperature according to the equation
logk = constant −Ea2.303R(1T)
where Ea is the energy of activation for the reaction . When a graph is plotted for log k versus 1T , a straight line with a slope - 6670 K is obtained. Calculate the energy of activation for this reaction
View SolutionThe rate constant 'k'. For a reaction varies with temperature 'T' according to the question. logk=logA−Ea2.303R(1T)
Where Ea is the activation energy. When a graph is plotted for logkvs1/T, a straight line with a slope of −4250 K is obtained. Calcualte Ea for this reaction.
View SolutionRate constant k of a reaction varies with temperature T according to the following Arrhenius equation.
logklogAEa2.303R(1T) where Ea is the activation energy . When a graph is plotted is or logk Vs 1T a straight line with a slope of −400K is obtained . Calculate the activation energy .
View SolutionConsider the given plots for a reaction obeying Arrhenius equation (0∘C<T<300∘C) : (k and Ea are rate constant and activation energy, respectively)
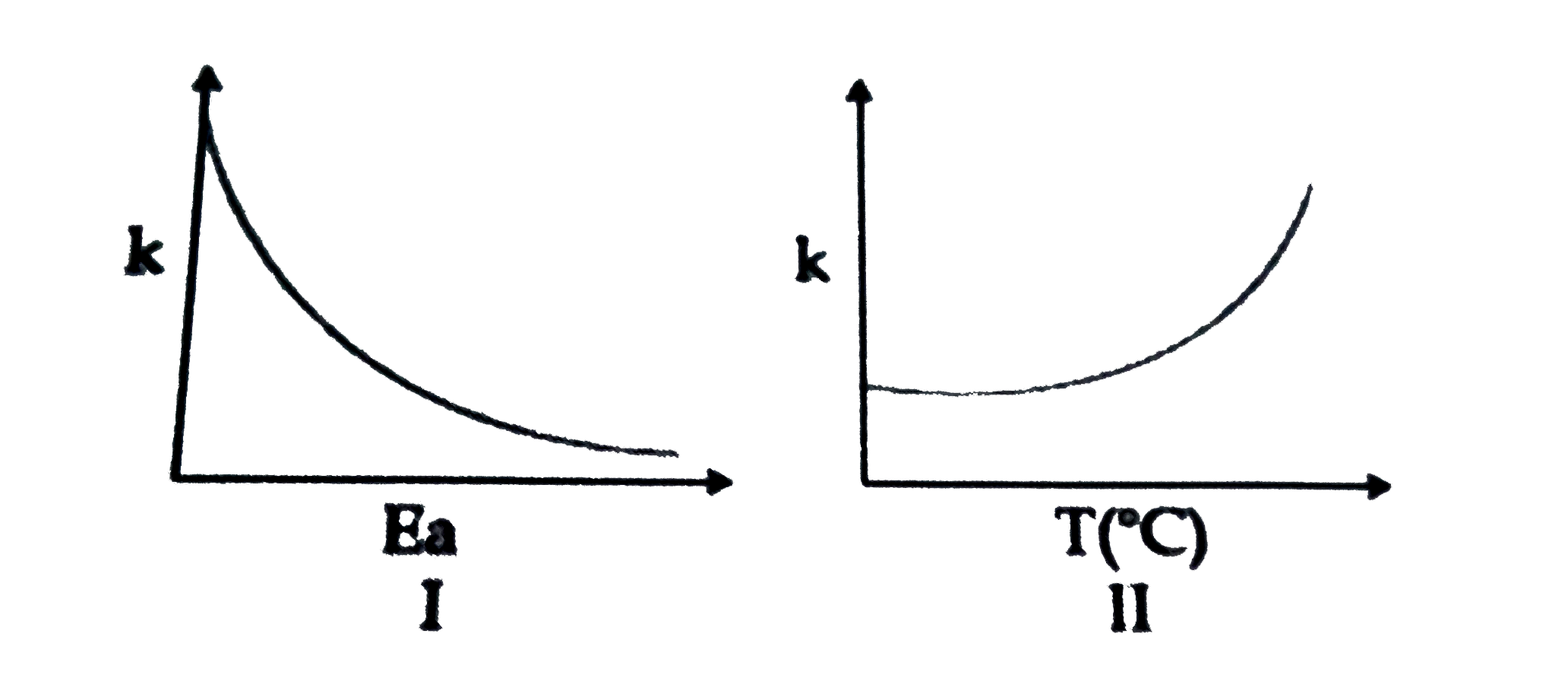
View Solutionनीचे दी गई आरेनियस समीकरण पर विचार करें तथा सही विकल्प पर निशान लगाएं।
k=Ae−Ea/RT
View Solutionआहेनियस समीकरण को मानने वाली एक अभिक्रिया के लिए आरेखों पर विचार कीजिए। (0∘C<T<300∘C), (k तथा Ea क्रमशः दर नियतांक तथा संक्रमण ऊर्जा हैं)
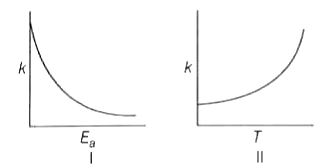
सही विकल्प चुनिए
View SolutionWrite Arrhenius equation relating activation energy (Ea),temperature(T) and rate const.(K).
View SolutionWrite Arrhenius equation relating activation energy (E_a) ,temperature(T) and rate const.(K).
View Solution

