A refrigerator converts 100 g of water at 25∘C into ice at −10∘C in one hour and 50 minutes. The quantity of heat removed per minute is (specific heat of ice = 0.5cal/g∘C, latent heat of fusion = 80cal/g) Solution in Gujrati
50cal
100 cal
200 cal
75 cal
The correct Answer is:B
Heat removed in cooling water from 25∘Cto0C=100×1×25=2500cal lthrgt Heat removed in converting water into ice at 0∘C=100×80=8000cal lthrgt Heat removed in cooling ice from 0∘Cto−10∘C=100×0.5×10=500cal
Total heat removed in 1hr50min=2500+8000+500=11000cal
Heat removed per minute = 11000110=100cal/min
Topper's Solved these Questions
- GEOMETRICAL OPTICSBook:ALLEN Chapter:GEOMETRICAL OPTICSExercise:EXERCISE -01
Explore 65 Videos
- GEOMETRICAL OPTICSBook:ALLEN Chapter:GEOMETRICAL OPTICSExercise:EXERCISE -02
Explore 82 Videos
- GEOMETRICAL OPTICSBook:ALLEN Chapter:GEOMETRICAL OPTICSExercise:subjective
Explore 14 Videos
- CURRENT ELECTRICITYBook:ALLEN Chapter:CURRENT ELECTRICITYExercise:EX.II
Explore 66 Videos
- GRAVITATIONBook:ALLEN Chapter:GRAVITATIONExercise:EXERCISE 4
Explore 9 Videos
Similar Questions
On an X temperature scale, water freezes at −125.0∘X and boils at 375.0∘X. On a Y temperature scale, water freezes at −70.0∘Y and boils at −30.0∘Y. The value of temperature on X-scale equal to the temperature of 50.0∘Y on Y-scale is
View SolutionA triangular plate has two cavities , one square and one rectangular as shown in figure . The plate is heated.
_E01_066_Q01.png)
View SolutionA prism of refractive index n1 & another prism of reactive index n2 are stuck together without a gap as shown in the figure.The angle of the prisms are as shown. n1&n2depend onλ,the wavelength of light according to n1=1.20+10.8×104λ2&n2=1.45+1.80×104λ2 where λ is in nm.
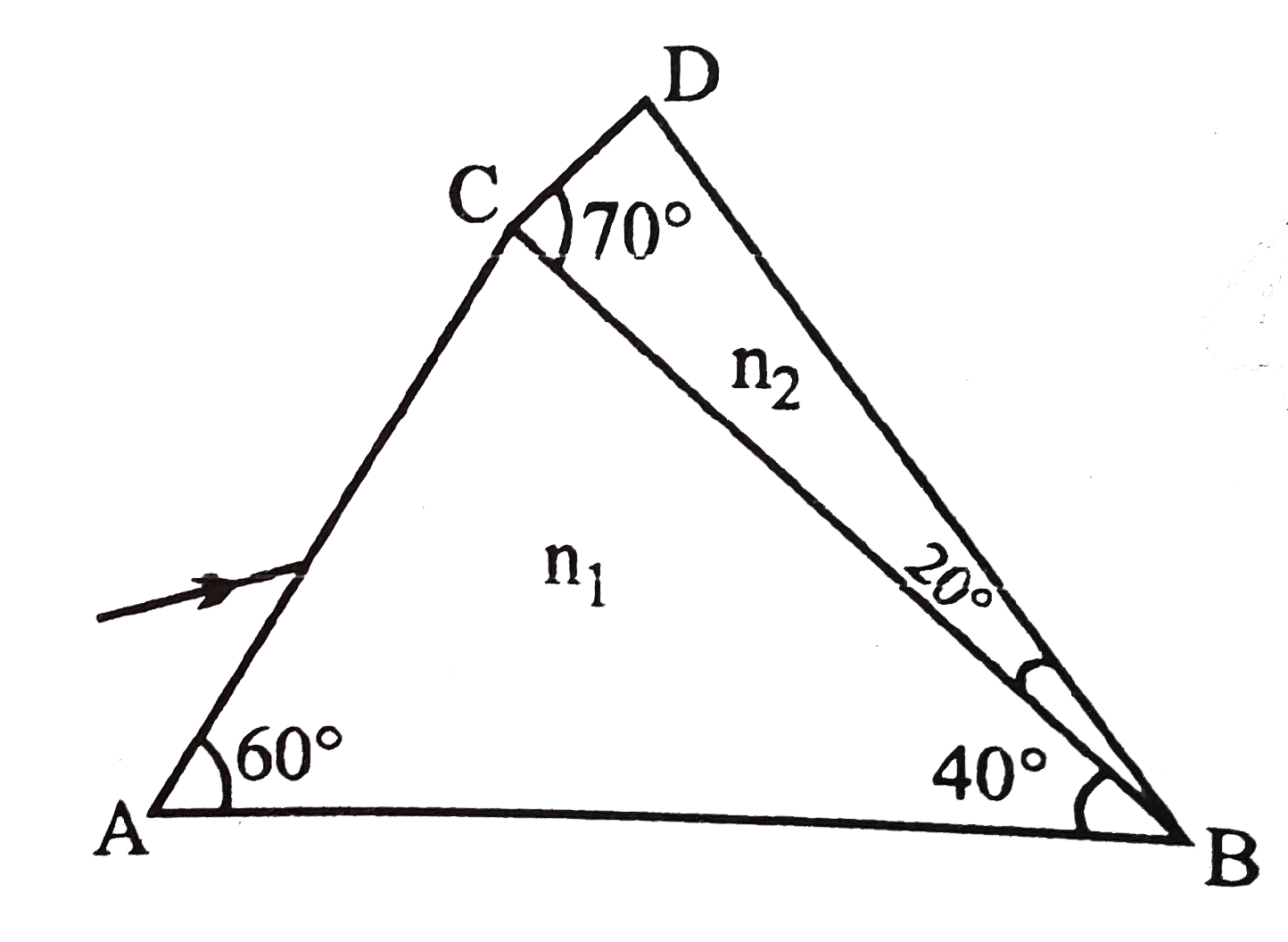
(i)Calculate the wavelength λ0for which rays incident at any angle on the interfaceBCpass through without bending at that interface.
(ii) for light of wavelength λ0,find the angle of incidencei on faceACsuch that the deviation produced by the combination of prism is minimum.
View SolutionIn the Bohr's model of hy drogen atom, the electron moves around the nucleus in a circular orbit of radius 5×10−11m. Its time period is 1.5×10−16s. The current associated with the electron motion is:-
View SolutionAssertion : The difference in the value of acceleration due to gravity at pole and equator is proportional to square of angular velocity of earth.
Reason : the value of acceleration due to gravity is minimum at the equator and maximum at the pole.
View SolutionA refrigeratior converts 50 gram of water at 15^(@)C intoice at 0^(@)C in one hour. Calculate the quantity of heat removed per minute. Take specific heat of water =1 cal g^(-1) .^(@)C^(-1) and latent heat of ice =80 cal g^(-1)
View Solution120 g of ice at 0^(@)C is mixed with 100 g of water at 80^(@)C . Latent heat of fusion is 80 cal/g and specific heat of water is 1 cal/ g-.^(@)C . The final temperature of the mixture is
View SolutionWhat amount of ice will remains when 52 g ice is added to 100 g of water at 40^(@)C ? Specific heat of water is 1 cal/g and latent heat of fusion of ice is 80 cal/g.
View SolutionCalculate the amount of heat required to convert 80g of ice at 0∘C into water at the same temperature . ( Specific latent heat of fusion of ice =80cal/g )
View SolutionFind the heat required to convert 20 g of iceat 0∘C into water at the same temperature. ( Specific latent heat of fusion of ice =80cal/g )
View Solution50 g of ice at 0^@C is mixed with 50 g of water at 80^@C . The final temperature of the mixture is (latent heat of fusion of ice =80 cal //g , s_(w) = 1 cal //g ^@C)
View Solution100 gm of water at 20∘C is converted into ice at 0∘C by a refrigerator in 2 hours. What will be the quantity of heat removed per minute? Specific heat of water = 1calg−1C−1 and latent heat of ice =80calg−1
View Solution20g of steam at 100∘C is passed into 100g of ice at 00C. Find the resultant temperature if specific latent heat of steam is 540 callg., specific latent heat of ice is 80 cal/g and specific heat of water is 1 cal/g0 C .
View SolutionThe amount of heat required to raise the temperature of 75 kg of ice at 0°C to water at 10°C is (latent heat of fusion of ice is 80 cal/g, specific heat of water is 1 cal/g °C)
View SolutionCalculate the amount of heat required to convert 10g of ice at - 10∘C to water at 5∘C (latent heat of fusion of ice = 80 cal g−1, specific heat of ice = 0.5).
View Solution120 g of ice at 0^(@)C is mixed with 100 g of water at 80^(@)C . Latent heat of fusion is 80 cal/g and specific heat of water is 1 cal/ g-.^(@)C . The final temperature of the mixture is
View Solution
