Which of the following is aromatic ?

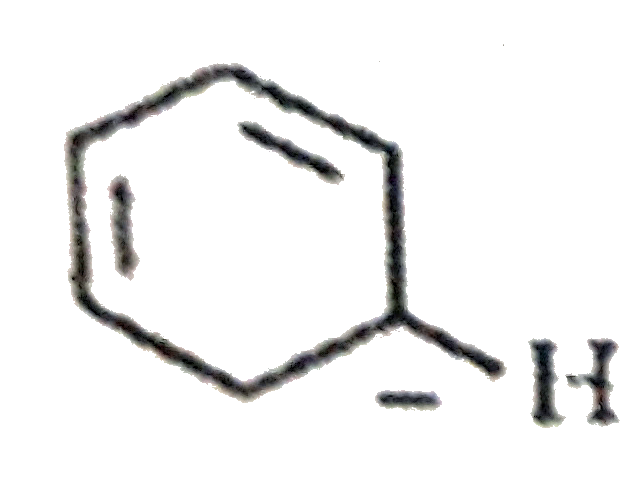
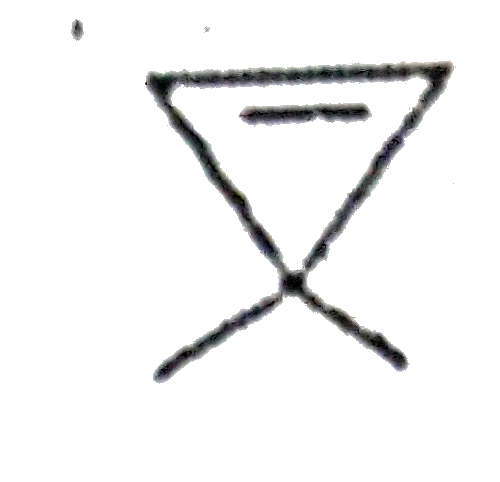
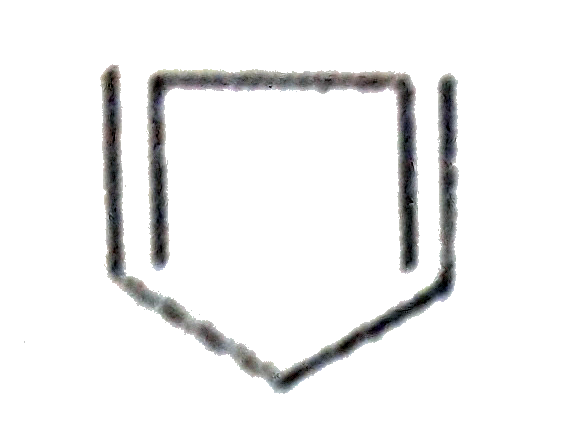
The correct Answer is:B
To determine which of the given compounds is aromatic, we need to apply the criteria for aromaticity, which are based on Huckel's rule. Here’s a step-by-step solution:
Step 1: Identify the Criteria for Aromaticity
A compound must meet the following four criteria to be considered aromatic:
1. Cyclic Structure: The compound must be cyclic.
2. Planarity: The compound must be planar (2D).
3. Complete Conjugation: The compound must have a complete conjugated system of alternating single and double bonds.
4. Hückel's Rule: The compound must have (4n + 2) π electrons, where n is a non-negative integer (0, 1, 2, ...).
Step 2: Analyze Each Compound
Now, we will analyze each of the given compounds based on the criteria.
Compound 1:
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If it has alternating double and single bonds and no interruptions, it is conjugated. If it has lone pairs or breaks in the conjugation, it is not.
- Hückel's Rule: Count the π electrons. If it does not meet the (4n + 2) rule, it is non-aromatic.
Conclusion: This compound is non-aromatic due to lack of complete conjugation.
Compound 2:
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If there are lone pairs or breaks in the conjugation, it is not conjugated.
- Hückel's Rule: Count the π electrons.
Conclusion: This compound is also non-aromatic due to lack of complete conjugation.
Compound 3:
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If it is not fully conjugated, it will not be aromatic.
- Hückel's Rule: Count the π electrons.
Conclusion: This compound is non-aromatic due to lack of complete conjugation.
Compound 4:
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If it has interruptions in conjugation, it is not aromatic.
- Hückel's Rule: Count the π electrons.
Conclusion: This compound is non-aromatic due to lack of complete conjugation.
Final Conclusion
After analyzing all the compounds, we find that all of them do not meet the criteria for aromaticity. Therefore, all options are non-aromatic.
---
A compound must meet the following four criteria to be considered aromatic:
1. Cyclic Structure: The compound must be cyclic.
2. Planarity: The compound must be planar (2D).
3. Complete Conjugation: The compound must have a complete conjugated system of alternating single and double bonds.
4. Hückel's Rule: The compound must have (4n + 2) π electrons, where n is a non-negative integer (0, 1, 2, ...).
Now, we will analyze each of the given compounds based on the criteria.
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If it has alternating double and single bonds and no interruptions, it is conjugated. If it has lone pairs or breaks in the conjugation, it is not.
- Hückel's Rule: Count the π electrons. If it does not meet the (4n + 2) rule, it is non-aromatic.
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If there are lone pairs or breaks in the conjugation, it is not conjugated.
- Hückel's Rule: Count the π electrons.
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If it is not fully conjugated, it will not be aromatic.
- Hückel's Rule: Count the π electrons.
- Cyclic: Yes, it is cyclic.
- Planar: Yes, it is planar.
- Conjugation: Check the bonding structure. If it has interruptions in conjugation, it is not aromatic.
- Hückel's Rule: Count the π electrons.
After analyzing all the compounds, we find that all of them do not meet the criteria for aromaticity. Therefore, all options are non-aromatic.
Similar Questions
Which one of the following will be aromatic?
View Solutionनिम्नलिखित में से कौन एरोमैटिक नहीं है
View Solutionनिम्न में से कौन-सी प्रजाति एरोमेटिक है
View Solutionनिम्न में से कौन-सी स्पीशीज ऐरोमैटिक है?
View Solutionनिम्न में से कौन एरोमैटिक यौगिक है ?
View SolutionWhich of the following are not aromatic?
View SolutionWhich of the following compounds are aromatic?
View SolutionWhich of the following species is aromatic :
View Solution

