Which of the following is optically inactive ?
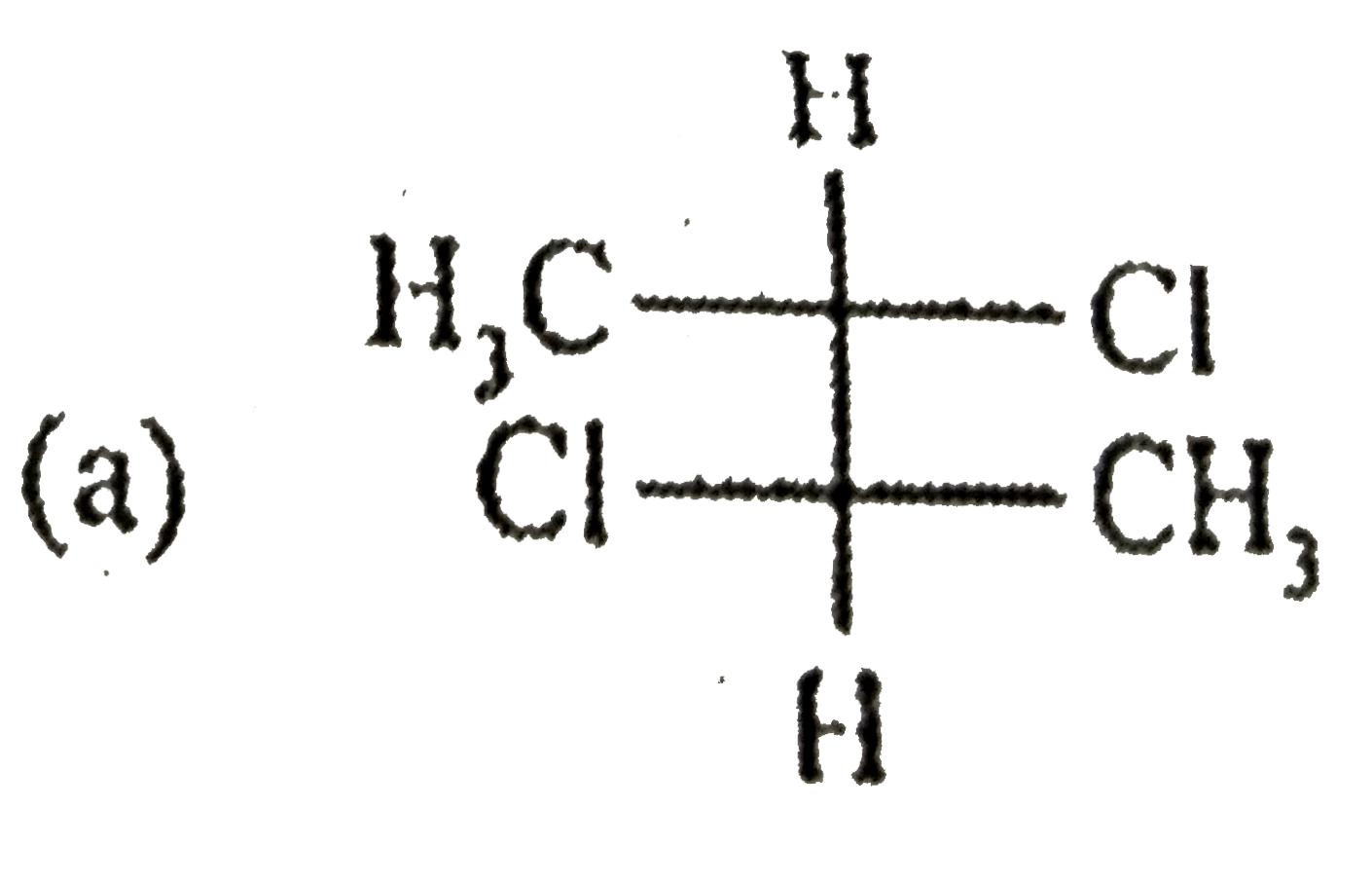
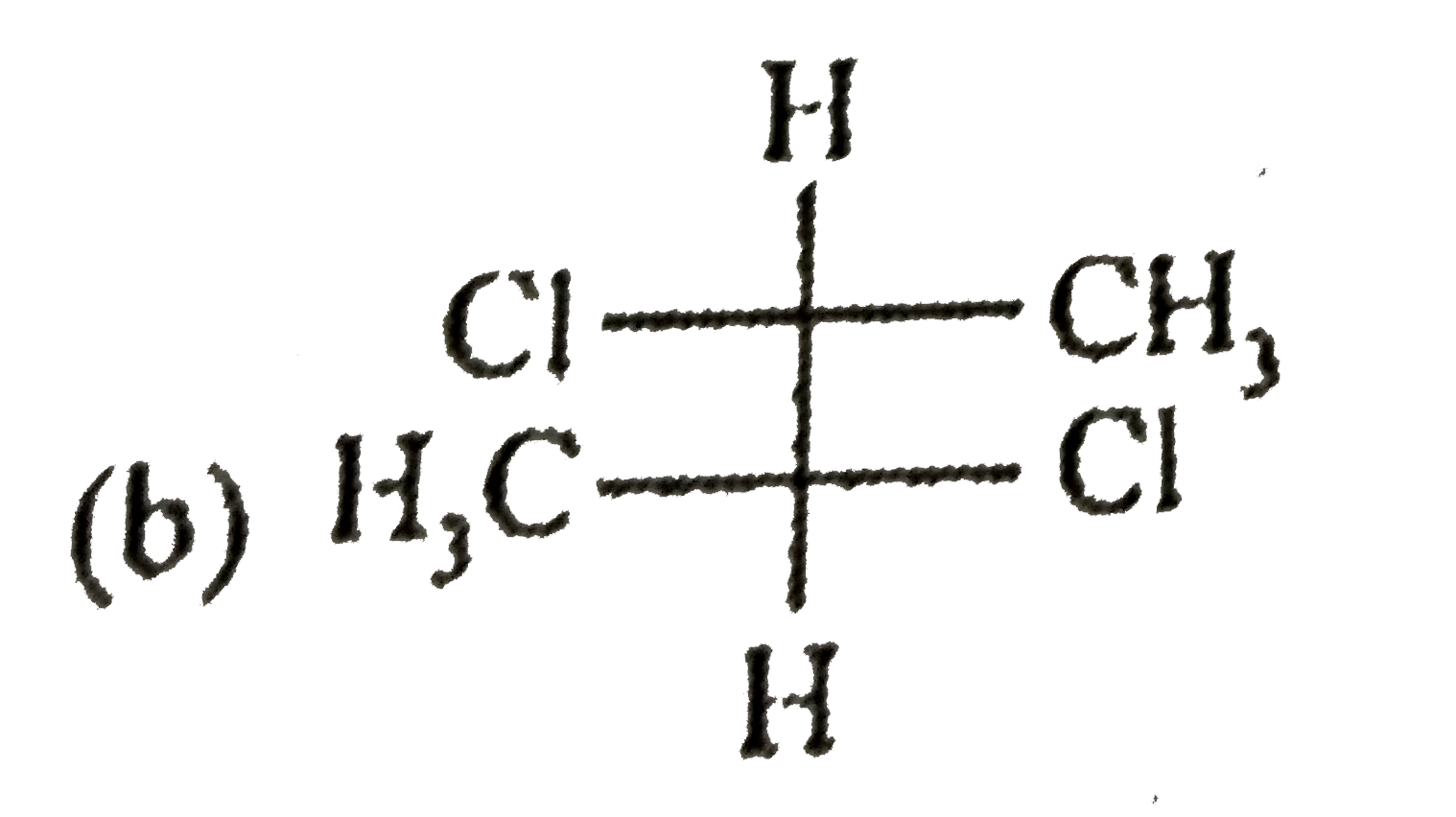
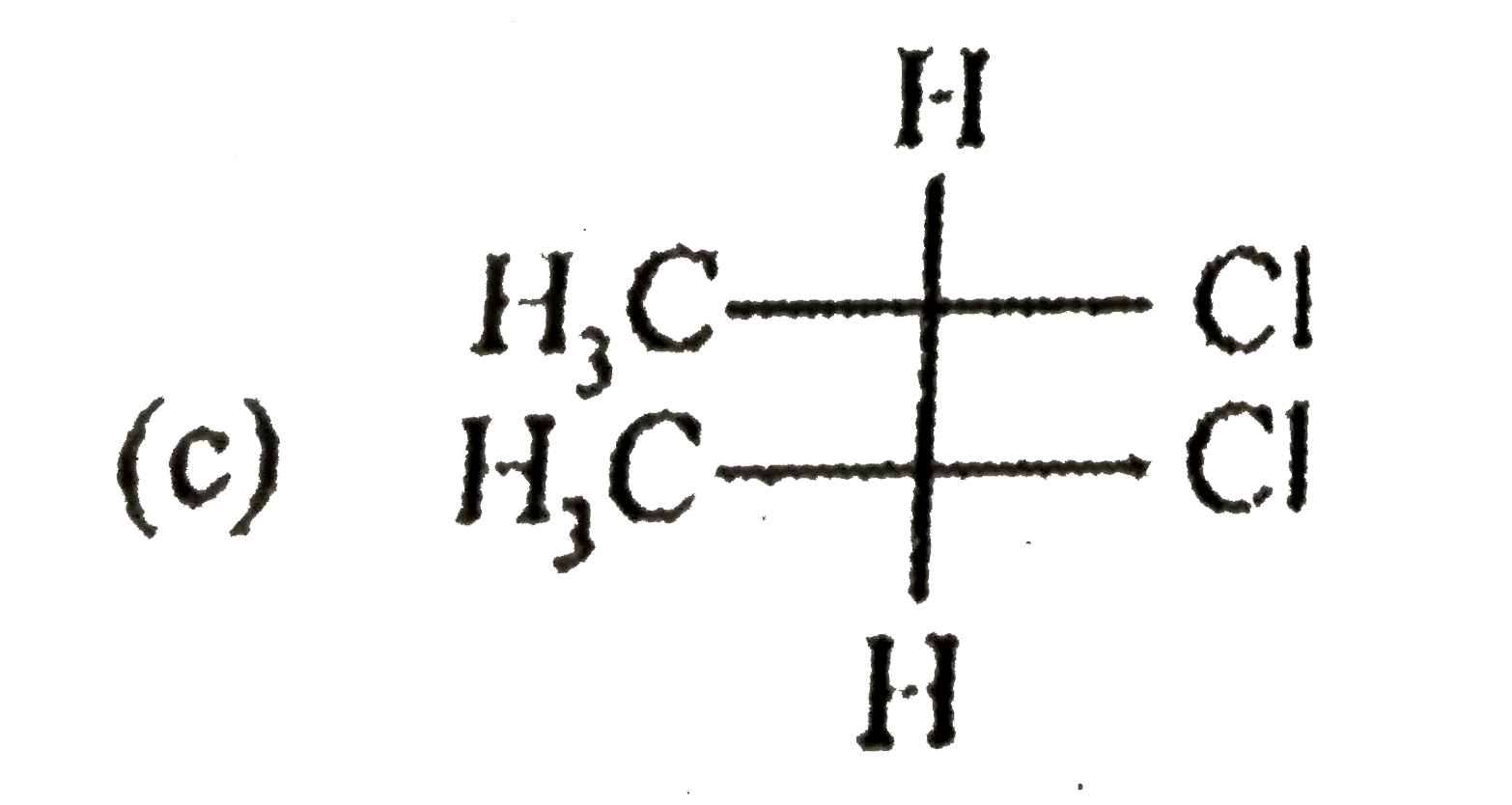
none of these
The correct Answer is:C
To determine which of the given compounds is optically inactive, we need to identify the characteristics of optically inactive compounds. Optically inactive compounds are typically achiral, meaning they do not have a chiral center or they possess a plane of symmetry that makes them superimposable on their mirror images.
1. Identify Chiral Centers:
- A chiral center is a carbon atom that is bonded to four different groups. If a compound has one or more chiral centers, it is likely to be optically active unless it has a plane of symmetry.
2. Examine Each Compound:
- For each compound provided in the options, we will analyze the structure to see if there are any chiral centers.
- If a compound has no chiral centers, it is automatically optically inactive.
3. Look for a Plane of Symmetry:
- If a compound has a plane of symmetry, it can be classified as a meso compound, which is also optically inactive despite having chiral centers.
4. Evaluate the Given Options:
- Option A: Analyze the structure. If it has chiral centers with no plane of symmetry, it is optically active.
- Option B: Similarly, check for chiral centers and symmetry.
- Option C: If this compound has a plane of symmetry, it is a meso compound and thus optically inactive.
- Option D: Check for chiral centers and symmetry.
5. Conclusion:
- After analyzing all the options, if Option C is determined to have a plane of symmetry, it is the correct answer as it is optically inactive.
Final Answer:
The optically inactive compound is Option C.
- A chiral center is a carbon atom that is bonded to four different groups. If a compound has one or more chiral centers, it is likely to be optically active unless it has a plane of symmetry.
- For each compound provided in the options, we will analyze the structure to see if there are any chiral centers.
- If a compound has no chiral centers, it is automatically optically inactive.
- If a compound has a plane of symmetry, it can be classified as a meso compound, which is also optically inactive despite having chiral centers.
- Option A: Analyze the structure. If it has chiral centers with no plane of symmetry, it is optically active.
- Option B: Similarly, check for chiral centers and symmetry.
- Option C: If this compound has a plane of symmetry, it is a meso compound and thus optically inactive.
- Option D: Check for chiral centers and symmetry.
- After analyzing all the options, if Option C is determined to have a plane of symmetry, it is the correct answer as it is optically inactive.
The optically inactive compound is Option C.
Similar Questions
Knowledge Check
Which of the following are optically active?
A B
B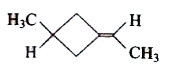 CCH3−CH∣D−COOHD
CCH3−CH∣D−COOHD
Which of the following are optically active?
A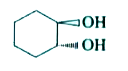 B
B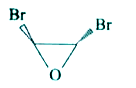 C
C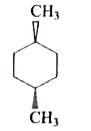 D
D
Which of the following is an optically inactive compound?
View SolutionWhich of the following is not optically active?
View SolutionWhich of the following is not optically active?
View SolutionWhich of the following is/are optically active?
View SolutionWhich of the following be optically active ?
View SolutionWhich of the following is chemically inactive allotropic form of carbon ?
View Solution

