The reaction of an organic compound with ammonia followed by nitration of the product gives a powerful explosive called RDX. The organic compound is
phenol
toluene
glyecrine
fomaldehyde.
The correct Answer is:B
To solve the question regarding the organic compound that reacts with ammonia and subsequently undergoes nitration to form RDX, we can follow these steps:
1. Identify the Final Product (RDX):
RDX, or Research Department Explosive, is a well-known powerful explosive. It is derived from the nitration of certain organic compounds.
2. Understand the Reaction Pathway:
The question indicates that the organic compound first reacts with ammonia and then undergoes nitration. This suggests that the compound must be one that can react with ammonia to form an intermediate product suitable for nitration.
3. Consider Common Organic Compounds:
A common organic compound that can react with ammonia is toluene. Toluene (C7H8) is a methyl-substituted benzene and is known to undergo various chemical reactions, including nitration.
4. Reaction of Toluene with Ammonia:
When toluene reacts with ammonia, it can form an aniline derivative. This step is crucial as it prepares the compound for the next nitration step.
5. Nitration of the Product:
The aniline derivative formed from toluene can then undergo nitration. Nitration typically involves the introduction of nitro groups (-NO2) into the aromatic ring. In the case of toluene, this can lead to the formation of trinitrotoluene (TNT).
6. Formation of RDX from TNT:
TNT can be further processed to produce RDX. The nitration of TNT introduces additional nitro groups, enhancing its explosive properties and resulting in RDX.
7. Conclusion:
Based on the above reasoning, the original organic compound that reacts with ammonia and subsequently undergoes nitration to form RDX is toluene.
Final Answer:
The organic compound is toluene.
---
RDX, or Research Department Explosive, is a well-known powerful explosive. It is derived from the nitration of certain organic compounds.
The question indicates that the organic compound first reacts with ammonia and then undergoes nitration. This suggests that the compound must be one that can react with ammonia to form an intermediate product suitable for nitration.
A common organic compound that can react with ammonia is toluene. Toluene (C7H8) is a methyl-substituted benzene and is known to undergo various chemical reactions, including nitration.
When toluene reacts with ammonia, it can form an aniline derivative. This step is crucial as it prepares the compound for the next nitration step.
The aniline derivative formed from toluene can then undergo nitration. Nitration typically involves the introduction of nitro groups (-NO2) into the aromatic ring. In the case of toluene, this can lead to the formation of trinitrotoluene (TNT).
TNT can be further processed to produce RDX. The nitration of TNT introduces additional nitro groups, enhancing its explosive properties and resulting in RDX.
Based on the above reasoning, the original organic compound that reacts with ammonia and subsequently undergoes nitration to form RDX is toluene.
The organic compound is toluene.
Similar Questions
The reaction of an organic compound with ammonia followed by nitration of the product gives a powerful explosive, called RDX. The organic compound is
View SolutionThe consituent of the powerful explosive RDX is formed during the nitration of
View SolutionGive only reaction for detection of nitrogen in organic compound
View SolutionGive only reaction for detection of nitrogen in organic compound
View SolutionAn organic compound on reaction with ozone followed by zinc and water gives
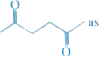 as the final product. The compound may be
as the final product. The compound may be
View SolutionAn organic compound undergoes addition reactions and gives a red precipitate with ammoniacal cuprous chloride. Therefore, the organic compound could be:
View SolutionGive the products of the following organic reactions :
View SolutionWhich of the following organic compounds unable to give precipitation after reaction with silver nitrate (AgNO3) ?
View Solution

