The experimental data for decomposition of
N2O5[2N2O5→4NO2+O2]
in gas phase at 318 K are given below:
(##KAL_KLC_ISC_CHE_XII_C04_SLV_030_Q01.png" width="80%">
Calculate the half life period fromk and compare with (b).
Half - life t1/2=0.693k=0.6934.8550×10−4s−1=1428s
Topper's Solved these Questions
- CHEMICAL KINETICSBOOK - ICSECHAPTER - CHEMICAL KINETICSEXERCISE - PROBLEM
165 Videos
- CHEMICAL KINETICSBOOK - ICSECHAPTER - CHEMICAL KINETICSEXERCISE - INTEXT QUESRTIONS
81 Videos
- BIOMOLECULESBOOK - ICSECHAPTER - BIOMOLECULESEXERCISE - ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)
21 Videos
- CHEMISTRY IN EVERYDAY LIFEBOOK - ICSECHAPTER - CHEMISTRY IN EVERYDAY LIFEEXERCISE - EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))
19 Videos
Similar Questions
From the data given below, calculate the average rate of the reaction :
C4H9Cl+H2O→C4H9OH+HCl during different intervals of time.
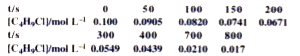
View SolutionWhat is reaction rate ?
View SolutionAn example of fibrous protein is :
View SolutionWhat is chemotherapy ? Describe the following briefly with one example in each case: analgesics.
View SolutionThe experimental data for decomposition of N2O5 in a gas phase at 318K are given below
2N2O5→4NO2+O2
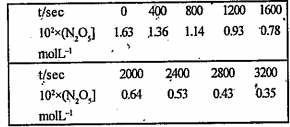
Calculate the half-life period from K
View SolutionThe experimental data for decomposition of N_2 O_5 [2 N_2 O_5 to 4NO_2 +O_2 ] in gas phase at 318 K are given below: (##KAL_KLC_ISC_CHE_XII_C04_SLV_025_Q01.png" width="80%"> Plot [N_2 O_5] against
View SolutionThe experimental data for decomposition of
N2O5[2N2O5→4NO2+O2]
in gas phase at 318 K are given below:
(##KAL_KLC_ISC_CHE_XII_C04_SLV_026_Q01.png" width="80%">
Find the half-life period for the reaction
View SolutionThe experimental data for decomposition of N_2 O_5 [2 N_2 O_5 to 4NO_2 +O_2 ] in gas phase at 318 K are given below: (##KAL_KLC_ISC_CHE_XII_C04_SLV_027_Q01.png" width="80%"> Draw a graph between log [N_2 O_5] and t .
View SolutionThe experimental data for decomposition of N_2 O_5 [2 N_2 O_5 to 4NO_2 +O_2 ] in gas phase at 318 K are given below: (##KAL_KLC_ISC_CHE_XII_C04_SLV_028_Q01.png" width="80%"> What is rate law?
View SolutionThe experimental data for decomposition of
N2O5[2N2O5→4NO2+O2]
in gas phase at 318 K are given below:
(##KAL_KLC_ISC_CHE_XII_C04_SLV_029_Q01.png" width="80%">
Calculate the rate constant.
View SolutionThe experimental data for decomposition of
N2O5[2N2O5→4NO2+O2]
in gas phase at 318 K are given below:
(##KALKLCISCCHEXIIC04SLV025_Q01.png width=80%><br>Plot[N_2 O_5] against
View SolutionThe experimental data for decomposition of
N2O5[2N2O5→4NO2+O2]
in gas phase at 318 K are given below:
(##KAL_KLC_ISC_CHE_XII_C04_SLV_026_Q01.png" width="80%">
Find the half-life period for the reaction
View SolutionThe experimental data for decomposition of
N2O5[2N2O5→4NO2+O2]
in gas phase at 318 K are given below:
(##KALKLCISCCHEXIIC04SLV027_Q01.png width=80%><br>Drawagraphbetweenlog [N_2 O_5] and t .
View SolutionThe experimental data for decomposition of N_2 O_5 [2 N_2 O_5 to 4NO_2 +O_2 ] in gas phase at 318 K are given below: (##KAL_KLC_ISC_CHE_XII_C04_SLV_028_Q01.png" width="80%"> What is rate law?
View SolutionThe experimental data for decomposition of N_2 O_5 [2 N_2 O_5 to 4NO_2 +O_2 ] in gas phase at 318 K are given below: (##KAL_KLC_ISC_CHE_XII_C04_SLV_029_Q01.png" width="80%"> Calculate the rate constant.
View Solution

