Dehydrobromination of compounds (A) and (B) yield the same alkene (C). Alkene (C) can regenerate (A) and (B) by the addition of HBr in the presence and absence of peroxide respectively. Hydrolysis of (A) and (B) give isomeric products (D) and (E) respectively. 1, 1-Diphenylethane is obtained on reaction of (C) with benzene in presence of H+. Give structures of (A) to (E) with reasons.
It is given that the compound C (alkene) on treatment with benzene in presence of H+ ion gives 1, 1- diphenylethane. Clearly, the compound (C) must be styrene. It is an example of Friedel-Craft reaction.
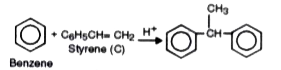
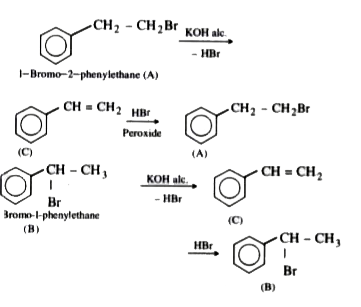
As the dehydrobromination of (A) and (B) give the same alkene (C). Clearly, (A) and (B) must be isomeric aralkyl halides. (C) on treatment with HBr in presence and absence of peroxide gives (A) and (B) respectively.
The structural formulae of (A) and (B) appear to be
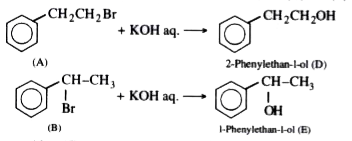
Hydrolysis of (A) and (B) with aq. KOH gives (D) and (E ) .
Also (C) reacts with benzene in presence of H+ to give 1, 1-Diphenylethane. It is Friedel-Craft reaction.
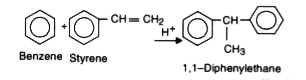
Topper's Solved these Questions
Similar Questions
Two different G.R.(A) and (B) give the product (X)
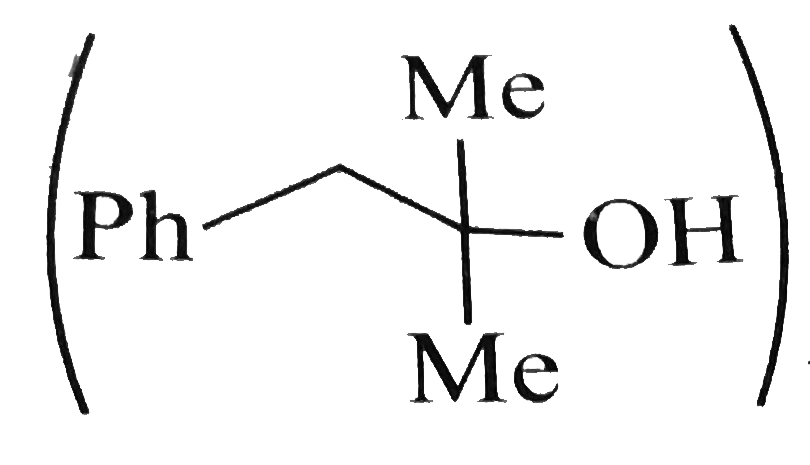 on reaction with (C) and (D) respectively. Give the structures of (A),(B),(C) and (D).
on reaction with (C) and (D) respectively. Give the structures of (A),(B),(C) and (D).
View SolutionWrite IUPAC names of the products obtained by addition reactions of HBr to hex-1-ene
(i) in the absence of peroxide and
(ii) in the presence of peroxide.
View Solution
Knowledge Check
The reaction intermediates involved in the addition of HBr to propene in the presence of an organic peroxide are
(a) free radicals
(b)carbocations
(c)carbanions
(d)carbenesAfree radicalsBcarbocationsCcarbanionsDcarbenes
In ABC, D and E are the mid-points of AB and AC respectively. Find the ratio of the areas of ADE and ABC .
View SolutionIn the following sequence of reactions, products A, B, C, D, and E are formed:

The structure of product (C) is:
View SolutionIn the following sequence of reactions, products A, B, C, D, and E are formed:

The structure of product (C) is:
View SolutionAn aliphatic unsaturated hydrocarbon (A) when treated with HgS∅H2SO4 yields a compound (B) having molecular formula C3H6O. (B) on oxidation with concentrated HNO3 gives two compounds (C) and (D). Compound (C) when treated with PCl5 gives compound (E). (E) when reacts with ethanol gives a sweet-smelling liquid (F). Compound (F) is also formed when (C) reacts with ethanol in the presence of concentrated H2SO4.
(i) Identify the compound A, B, C, D, E and F.
(ii) Give the chemical equation for the reaction of (C) with chlorine in the presence of red phosphorus and name the reaction.
View SolutionThe three compounds (A),(B),and (C) on hydrogenation with H2+Pd give (D),(E) and (F), respectively.
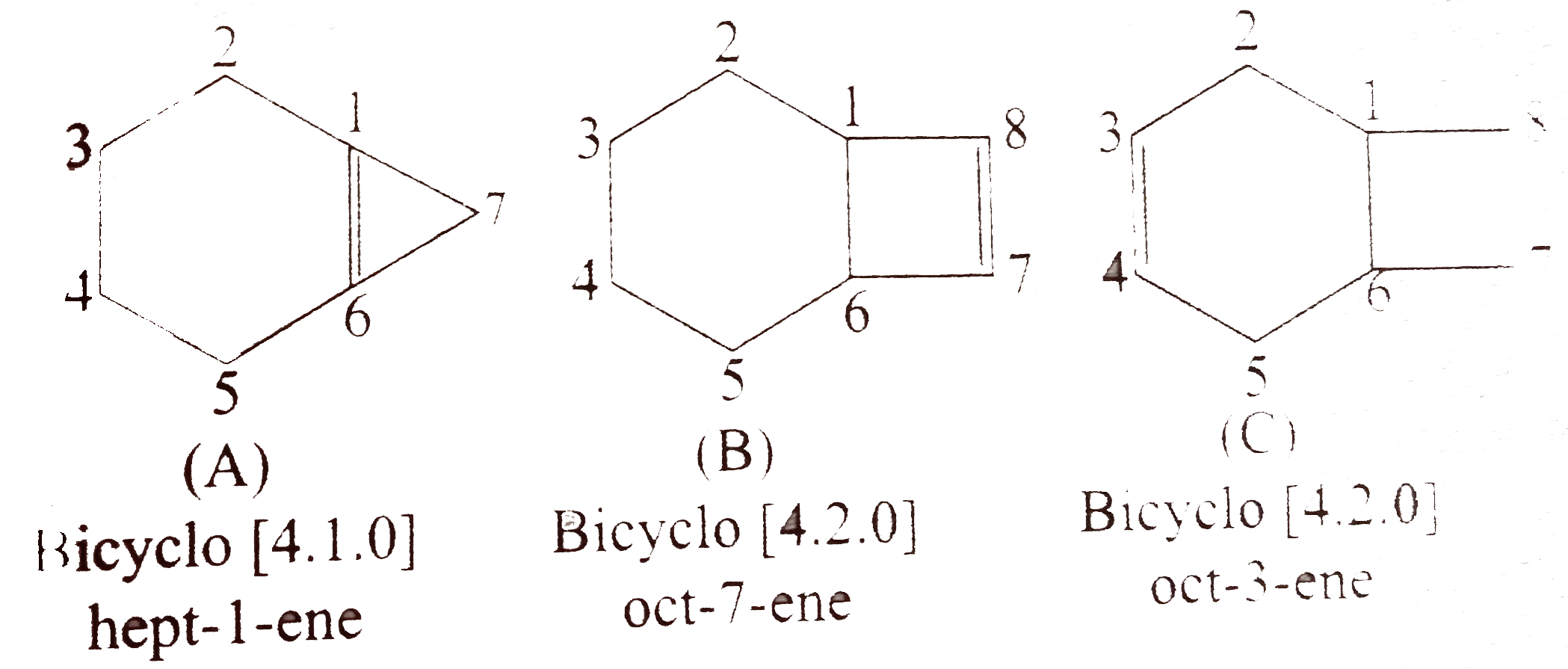
Compounds (D),(E) and (F) are :
View SolutionAn organic acid (A), C5H10O2 reacts with Br2 in the presence of phosphorous to give (B). Compound (B) contains an asymmetric carbon atom and yield (C) on dehydrabromination. Compound (C) does not show geometric isomerism and on decarboxylation give an alkene (D) which on ozonolysis gives (E) and (F), compound (E) gives a positive schiff's test but (F) does not. Give structures of (A) to (F) with reasons.
View SolutionTwo isomeric compounds, (A) and (B), have the same formula, C11H13OCI. Both are unsaturated, yield the same compound (C) on catalytic hydrogenation, and produce 4-chloro-3-ethoxybenzoic acid on vigorous oxidation. (A) exists in geometrical isomers, (D) and (E), but not (B). Give structures of (A) to (E) with proper reasoning.
View Solution

