Complete the following equations giving the names of the reactants and the products :
2HCHO+NaOH→
To complete the given equation and identify the reactants and products, we will follow these steps:
Step 1: Identify the Reactants
The reactants in the reaction are:
1. Formaldehyde (HCHO) - This is an aldehyde.
2. Sodium hydroxide (NaOH) - This is a strong base.
Step 2: Write the Balanced Equation
The reaction involves 2 moles of formaldehyde reacting with sodium hydroxide. The balanced equation can be written as:
2HCHO+NaOH→Products
Step 3: Identify the Type of Reaction
This reaction is known as the Cannizzaro reaction. It occurs when an aldehyde that lacks an alpha hydrogen (like formaldehyde) reacts with a strong base. In this reaction, one molecule of formaldehyde is oxidized while the other is reduced.
Step 4: Determine the Products
In the Cannizzaro reaction:
- One molecule of formaldehyde (HCHO) is oxidized to form sodium formate (HCOONa).
- The other molecule of formaldehyde is reduced to form methanol (CH3OH).
Thus, the products of the reaction are:
1. Sodium formate (HCOONa)
2. Methanol (CH3OH)
Step 5: Write the Complete Balanced Equation
Putting it all together, the complete balanced equation is:
2HCHO+NaOH→HCOONa+CH3OH
Summary of Reactants and Products
- Reactants:
- Formaldehyde (HCHO)
- Sodium hydroxide (NaOH)
- Products:
- Sodium formate (HCOONa)
- Methanol (CH3OH)
The reactants in the reaction are:
1. Formaldehyde (HCHO) - This is an aldehyde.
2. Sodium hydroxide (NaOH) - This is a strong base.
The reaction involves 2 moles of formaldehyde reacting with sodium hydroxide. The balanced equation can be written as:
2HCHO+NaOH→Products
This reaction is known as the Cannizzaro reaction. It occurs when an aldehyde that lacks an alpha hydrogen (like formaldehyde) reacts with a strong base. In this reaction, one molecule of formaldehyde is oxidized while the other is reduced.
In the Cannizzaro reaction:
- One molecule of formaldehyde (HCHO) is oxidized to form sodium formate (HCOONa).
- The other molecule of formaldehyde is reduced to form methanol (CH3OH).
1. Sodium formate (HCOONa)
2. Methanol (CH3OH)
Putting it all together, the complete balanced equation is:
2HCHO+NaOH→HCOONa+CH3OH
- Reactants:
- Formaldehyde (HCHO)
- Sodium hydroxide (NaOH)
- Products:
- Sodium formate (HCOONa)
- Methanol (CH3OH)
টপার এই প্রশ্নগুলো সমাধান করেছে
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:ISC EXAMINATION QUESTIONS (PART-I OBJECTIVE QUESTIONS) (FILL IN THE BLANKS)
Explore 27 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:ISC EXAMINATION QUESTIONS (PART-I OBJECTIVE QUESTIONS) (MULTIPLE CHOICE QUESTION)
Explore 24 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:ICSEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:EXERCISE (PART-II DESCRIPTIVE QUESTIONS) (VERY SHORT QUESTIONS)
Explore 282 Videos
- ALCOHOLS, PHENOLS AND ETHERS Book:ICSEChapter:ALCOHOLS, PHENOLS AND ETHERS Exercise:ISC EXAMINATION QUESTIONS
Explore 25 Videos
- APPENDIX II ( TYPICAL CONVERSIONS)Book:ICSEChapter:APPENDIX II ( TYPICAL CONVERSIONS)Exercise:AROMATIC CONVERSIONS
Explore 98 Videos
সম্পর্কিত প্রশ্ন
Complete the following equations giving the names of the reactants and the products :
CH3COCl+H2Pd/BaSO4−−−−−−→
View SolutionComplete the following equations giving the names of the reactants and the products :
(CH3COO)2CaΔ−−→
View SolutionComplete the following equations giving the names of the reactants and the products :
C6H5CHCl2+NaOH(aq)Heat−−→
View SolutionComplete the following equations giving the structures of the major organic product.
_E01_588_Q01.png)
View SolutionComplete the following equations :
PbO+NaOH→
View SolutionComplete the following reactions and name the products:

View SolutionComplete the following giving the structures of the principal organic products.
_E01_162_Q01.png)
View SolutionComplete the following equations :
Al2O3+NaOH→
View SolutionGive the products of the following and the name of the reactions:
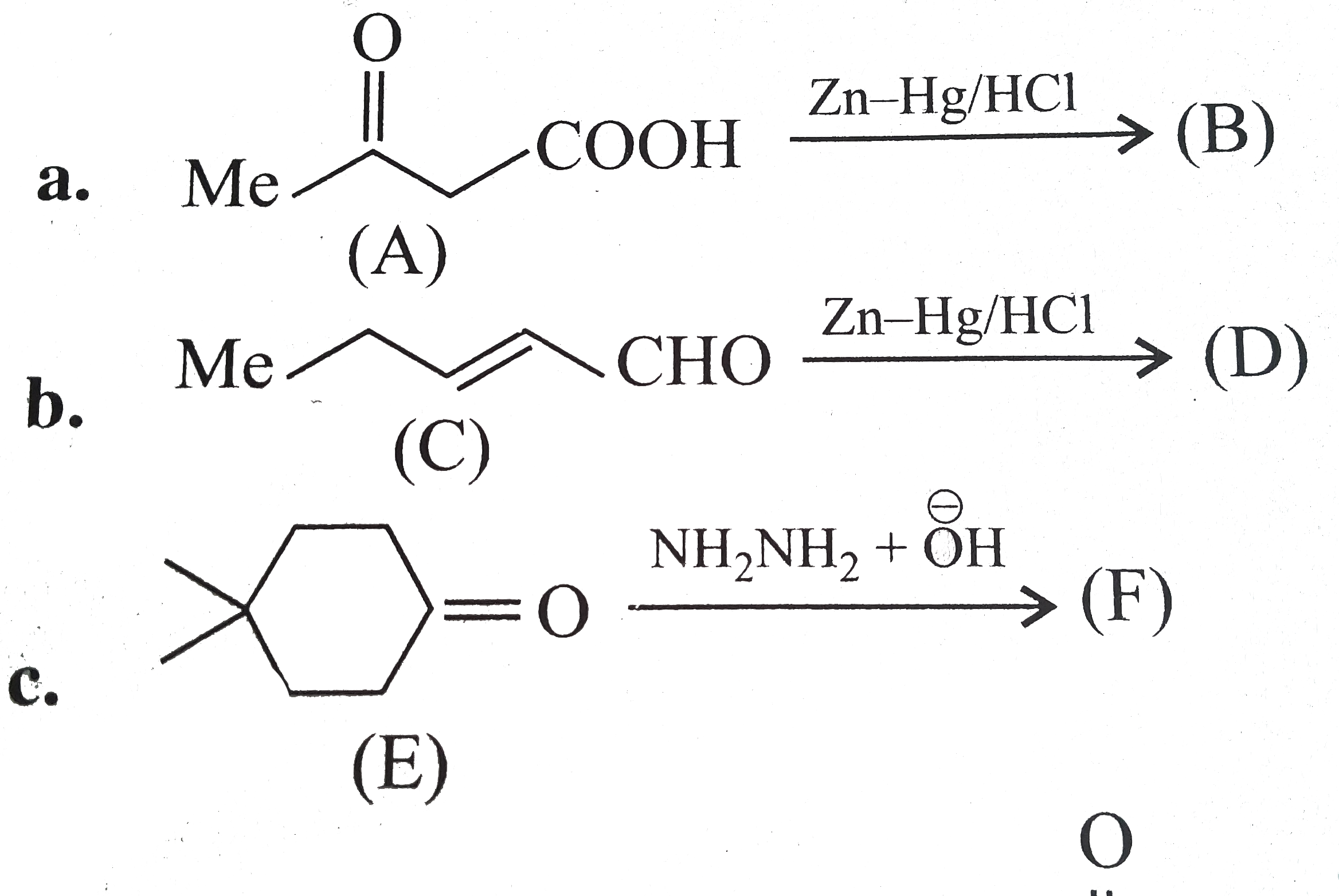
View SolutionComplete the following by giving the name of digestive juice/enzyme and the product formed in each case.
Protein :____
View Solution
