In general, it is observed that the rate of a chemical reaction doubles with every 10° rise in temperature. If this generalisation holds for a reaction in the temperature range 298 K to 308 K, what would be the value of activation energy for this reaction ? (R=8.314JK−1mol−1)
According to Arrhenius equation,
logK2k1=Eak1=Ea2.303R(T2−T1]T1T2
here T1=298K1=k (say ) T2=308K,K2=2k
∴log2kk=Ea2.303×8.314×[308−298298×308]
orEa=2.303×8.314×298×308×log210
=52903Jmol−1=52.9KJmol−1
Topper's Solved these Questions
- CHEMICAL KINETICSBOOK - ICSECHAPTER - CHEMICAL KINETICSEXERCISE - PROBLEM
165 Videos
- CHEMICAL KINETICSBOOK - ICSECHAPTER - CHEMICAL KINETICSEXERCISE - INTEXT QUESRTIONS
81 Videos
- BIOMOLECULESBOOK - ICSECHAPTER - BIOMOLECULESEXERCISE - ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)
21 Videos
- CHEMISTRY IN EVERYDAY LIFEBOOK - ICSECHAPTER - CHEMISTRY IN EVERYDAY LIFEEXERCISE - EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))
19 Videos
Similar Questions
From the data given below, calculate the average rate of the reaction :
C4H9Cl+H2O→C4H9OH+HCl during different intervals of time.
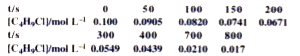
View SolutionWhat is reaction rate ?
View SolutionAn example of fibrous protein is :
View SolutionWhat is chemotherapy ? Describe the following briefly with one example in each case: analgesics.
View SolutionIn general, it is observed that the rate of a chemical reaction doubles with every 10∘ rise in temperature. If the generalisation holds for a reaction in the temperature range 295 K to 305 K, what would be the value of activation energy for the reaction?
View Solutionएक रासायनिक अभिक्रिया का ताप 290K से बढ़ाकर 300 K करने पर अभिक्रिया की दर दुगुनी हो जाती है। अभिक्रिया की सक्रियण ऊर्जा का मान ज्ञाज कीजिए।
(R=8.314JK−1mo−1,log102=0.3010)
View SolutionThe rate of most of reaction double when their temperature is raised from 298 K to 308 K. Calculate the activation energy of such a reaction.
View SolutionRates of reaction double with every 10^@ rise in temperature. If this generalization holds for a reaction in the temperature ranges 298 K to 308 K, what would be the value of activation energy for their reaction ? R = 8.314 J K^(-1) "mol"^(-1) .
View SolutionAssertion (A) : Rate of reaction will be doubled, when temperature increased from 298 k to 308 k.
Reason (R ): The activation energy of reaction decreases with increase in temperature.
View SolutionWhat is the activation energy for a reaction if its rate doubles when the temperature is raised from 200 K to 400 K ? (R=8.314JK−1mol−1)
View Solutionவெப்பநிலை 200 K இருந்து 400 K க்கு உயர்த்தப்படும் போது வினைவேகம் இரு மடங்கு அதிகரித்தால், கிளர்வு ஆற்றலின் மதிப்பு யாது? (R=8.314JK−1mol−1)
View SolutionIn general it is observed that the rate of a chemical reaction doubles with every 10 degree rise in temperature. If the generalization holds good for the reaction in the temperature range 295 K to 305 K, what would be the value of activation energy for this reaction ? [R=8.314mol−1JK−1]
View SolutionThe rate of the chemical reaction doubles for an increase of 10 K in absolute temperature from 298 K.Calculate Ea.
(R=8.314 J K1mol−1)
View SolutionIn general it is observed that the rate of a chemical reaction doubles with every 10∘ rise in temperature. If the generalisation holds for a reaction in the temperature range 295K to 305K, what would be the value of activation energy for the reaction?
View SolutionIn general it is observed that the rate of a chemical reaction doubles with every 10^(circ) rise in temperature. If this generalisation holds for a reaction in the temperature range 295 (~K) to 305 (~K) , what would be the value of activation energy for this reaction? (R)=8.314 (Jk)^(-1) (~mol)^(-1)
View Solution

